The evidence for LuPSMA in prostate cancer
Prostate cancer is the leading cause of death due to cancer in men.1,2 A typical example of theranostics, defined as a combination of therapy and diagnostic, is the use of radiolabeled ligands that bind to a specific target on cancer cells and emit radiation, allowing treatment planning, dosimetry and imaging using positron emission tomography (PET).3 Prostate-specific membrane antigen (PSMA) is a type II transmembrane-bound glycoprotein highly expressed in prostate cancer, particularly in poorly differentiated primary tumors and metastatic lesions.4–6 The PSMA-targeted radioligand 177Lutetium (Lu)-PSMA-617 (LuPSMA) has emerged as a promising therapeutic option for patients with advanced prostate cancer. LuPSMA is a small molecule that delivers high levels of β-radiation to PSMA-expressing cells. It is the first targeted radioligand therapy approved for the treatment of adult patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy,7 following the results from the open-label, phase III VISION trial.8
VISION was designed based on positive data from the randomized phase II TheraP trial that aimed to assess LuPSMA in patients with mCRPC for whom cabazitaxel was considered the next appropriate standard treatment.9 The study met its primary endpoint of prostate-specific antigen (PSA) response defined as a reduction of at least 50% from baseline, with patients in the LuPSMA arm (n=99) achieving significantly improved rates of PSA response versus those in the cabazitaxel arm (n=101) (66% vs 44%; p=0.0016). After a median follow-up of 3 years, overall survival (OS) was similar in patients assigned to LuPSMA versus cabazitaxel (restricted mean survival time [RMST], 19.1 months vs 19.6 months; HR: 0.97 [95% CI: 0.70−1.40]; p=0.99).10 However, LuPSMA significantly delayed progression versus cabazitaxel (HR: 0.62 [95% CI: 0.45−0.85]; p=0.0028), with RMST of 7.1 months versus 5.0 months, respectively.
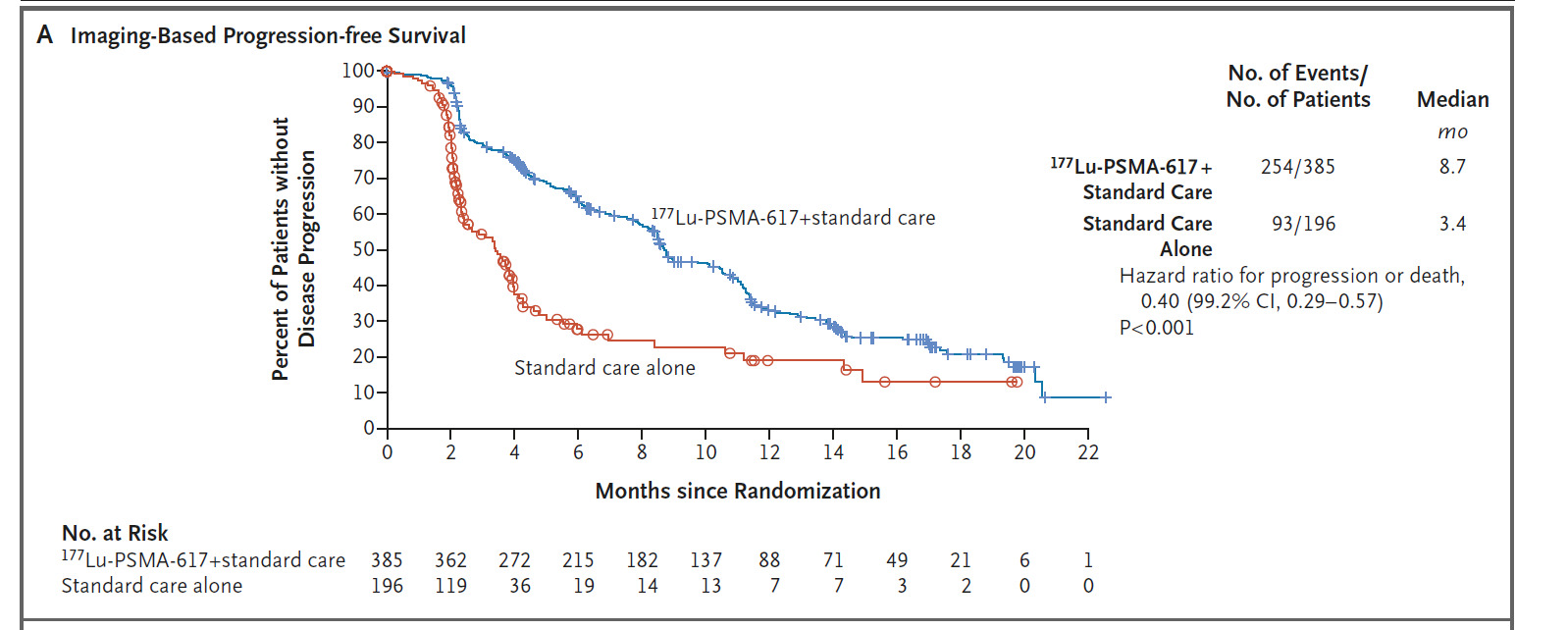
The registrational, open-label VISION trial further investigated LuPSMA in patients with mCRPC previously treated with ≥1 androgen receptor-pathway inhibitor (ARPI) and 1−2 taxane regimens and who had PSMA-positive 68Ga-PSMA-11 PET/CT scans.8 In total, 831 patients were randomized 2:1 to receive either LuPSMA (7.4 GBq every 6 weeks for 4–6 cycles) plus protocol-permitted standard of care (SoC) or SoC alone. At a median follow-up of 20.9 months, LuPSMA plus SoC versus SoC alone significantly improved radiographic progression-free survival (PFS) (median: 8.7 months vs 3.4 months; HR: 0.40 [99.2% CI: 0.29–0.57]; p<0.001) (Figure 1) and OS (median: 15.3 months vs 11.3 months; HR: 0.62 [95% CI: 0.52–0.74]; p<0.001). Among patients with measurable target lesions (n=248), 51% achieved a partial response or complete response in the Lu-PSMA plus SoC arm compared with 3% in the SoC alone arm.
Further analyses of VISION showed that LuPSMA plus SoC delayed time to worsening in health-related quality of life (HRQoL) compared with SoC alone.11 More specifically, time to worsening was delayed with LuPSMA-based treatment versus SoC alone for FACT-P score (HR: 0.54) and subdomains, BPI-SF pain intensity score (HR: 0.52) and EQ-5D-5L utility score (HR: 0.65). LuPSMA was also associated with prolonged time to first skeletal event or death (median, 11.5 months vs 6.8 months with SoC alone; HR: 0.50 [95% CI: 0.40–0.62]).
The safety profile of LuPSMA was generally manageable.8 The incidence of hematologic adverse events (AEs) of grade ≥3 during treatment was higher in the LuPSMA arm than in the control group and most commonly included anemia (12.9% vs 4.9%), thrombocytopenia (7.9% vs 1.0%), lymphopenia (7.8% vs 0.5%) and leukopenia (2.5% vs 0.5%). LuPSMA was also associated with low salivary gland and renal toxicity (grade ≥3: 0% and 3.4%). Data further showed that the blood counts and the creatinine levels remained stable over time under LuPSMA treatment.11
Predictors of response to LuPSMA
Theranostics can provide more precise and individualized treatment decisions in mCRPC. Prognostic nomograms have been developed to predict outcomes after LuPSMA therapy in patients with mCRPC by using both clinical trial and real-world data.12 Based on multivariate analysis, various characteristics were identified that predict OS outcomes with LuPSMA, including more traditional characteristics, such as age, time since diagnosis, hemoglobin level and chemotherapy status, and variables relevant in this patient population, including tumor standardized uptake value (SUV), number and site of metastatic lesions. By incorporating these data, patients were stratified into low-risk versus high-risk groups, with a median OS of 24.9 months versus 7.4 months (p<0.0001), respectively, in the validation cohort. Results further showed that patients with a higher PSMA expression had more favorable clinical outcomes, while those with bone involvement were less likely to benefit from LuPSMA therapy.
These results have been confirmed in a VISION substudy which aimed to assess the prognostic value of baseline 68Ga-PSMA-11 PET imaging in men undergoing LuPSMA therapy.13 Data from this analysis support the use of quantitative PSMA-PET imaging as a prognostic tool, showing a strong link between higher whole-body SUVmean and improved treatment outcomes with LuPSMA in patients with prostate cancer. Patients in the highest versus lowest SUVmean quartile had a longer radiographic PFS (median, 14.1 months vs 5.8 months) and OS (median, 21.4 months vs 14.5 months). These results suggest that patients with a higher SUVmean identified by 68Ga-PSMA-11 benefit most from LuPSMA therapy.
Recently, a novel framework called Response Evaluation Criteria In PSMA Imaging (RECIP) has been proposed for treatment response evaluation using PSMA-PET/computed tomography (CT) in patients with metastatic prostate cancer.14 RECIP 1.0 was developed using the appearance of new lesions and changes in PSMA-positive tumor volume (PSMA-VOL). Median OS by RECIP response was significantly lower in the RECIP-PD group (defined as an increase ≥20% in PSMA-VOL and appearance of new lesions) compared with the RECIP-PR group (decline ≥30% in PSMA-VOL and no appearance of new lesions) and RECIP-SD group (any other condition) (median OS of 8.3 months vs 21.7 months and 13.1 months; p<0.001). These data suggest that PSMA-PET/CT by RECIP 1.0 is prognostic for OS and can be used as a response biomarker to monitor the early efficacy of LuPSMA in mCRPC patients.
Emerging LuPSMA-based treatment regimens
Further studies have provided evidence that LuPSMA is an effective treatment option for mCRPC, both in earlier lines of therapy (PSMAFour15 and ENZA-p16 studies) and after treatment with radium 223 (RALU17,18). Ongoing clinical studies are currently assessing LuPSMA also in patients with metastatic hormone-sensitive prostate cancer (mHSPC)19,20 and as a neoadjuvant treatment.21 Several trials evaluated the safety and efficacy of LuPSMA in combination with other agents including abiraterone acetate,22 olaparib,23 enzalutamide,24 and pembrolizumab.25
Furthermore, other radiolabeled PSMA-targeting small molecules are being investigated as an alternative to 177LuPSMA-617. PSMA I&T,26 which contains a DOTAGA chelator (whereas LuPSMA-617 contains a DOTA chelator), demonstrated favorable safety in mCRPC patients27 and in high-risk localized prostate cancer before robot-assisted radical prostatectomy (RARP).28 The ongoing phase III SPLASH study29 evaluates PSMA I&T in mCRPC patients after second-line hormonal treatment. The primary completion of the trial is expected in December 2023. The derivative of LuPSMA-617 labeled with actinium-225 (225Ac-PSMA-617) has shown remarkable therapeutic efficacy in patients with mCRPC.30,31 The phase I/II VIOLET trial is currently recruiting patients to evaluate PSMA I&T labeled with terbium-161 (161Tb-PSMA I&T) in mCRPC.32
Conclusions
-
LuPSMA therapy has demonstrated prolonged survival in patients with mCRPC in multiple clinical trials, with favorable safety and considerably improved patient-reported outcomes.
-
LuPSMA therapy is currently approved for use in the third-line setting, after new hormonal therapy and chemotherapy in patients with mCRPC.
-
PSMA imaging can be used for precision oncology to identify patients for LuPSMA therapy and assess their response to treatment.
Conflicts of interest
The author has declared that the article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.