The never-ending story of fractionation
Dose-escalated radiation therapy (70−80 Grey [Gy]) delivered with conventional fractionation (1.8 or 2.0 Gy in 5 consecutive days per week) over 8−9 weeks has been the standard treatment of prostate cancer for years.1–4 However, radiobiological models showed that hypofractionated prostate radiotherapy may not only shorten the duration of treatment but also increase prostate cancer control by delivering of fewer and larger daily doses of radiation.11,12 In addition, shorter hypofractionated treatment is more convenient for the patient and associated with decreased treatment costs.13,14
Moderate hypofractionation versus conventional fractionation in patients with prostate cancer was assessed in several randomized phase III trials. Generally, hypofractionation showed a slightly increased incidence of acute gastrointestinal (GI) and genitourinary (GU) adverse events (AEs) but with no differences in long-term toxicity.15–20 Overall, results from these trials indicated that hypofractionated radiotherapy is safe and should be considered as the new standard of care for patients with localized prostate cancer. The most commonly used schedule was tested and established in the randomized CHHiP-trial, testing 20 fractions of 3 Gy versus 37 fractions of 2 Gy.
In contrast to moderate hypofractionation, ultra-hypofractionation, also referred to as extreme hypofractionation, stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy, uses fraction sizes of at least 5 Gy.21 This allows for shorter treatment courses but might be associated with increased acute toxicity compared with conventionally fractionated radiotherapy. Ultra-hypofractionated (42.7 Gy in 7 fractions, 3 days per week for 2.5 weeks) versus conventional fractionated (78.0 Gy in 39 fractions, 5 days per week for 8 weeks) radiotherapy was initially assessed in the non-inferiority, phase III HYPO-RT-PC trial in 1,180 patients with intermediate- and high-risk prostate cancer.22 At a median follow-up of 5 years, the primary endpoint of estimated failure-free survival was 84% in both treatment groups (adjusted HR: 1.002 [95% CI: 0.758−1.325]; p=0.99) (Figure 1). In terms of safety, there was a trend toward an increased rate of early GU AEs with ultra-hypofractionation compared with conventional fractionation at the end of radiotherapy (grade ≥2: 28% vs 23%; p=0.057), but late urinary toxicity (at 5 years) was similar in both treatment groups (grade ≥2: 5% each). In addition, there was no difference in late GI toxicities between the two treatment arms.
Another phase III, non-inferiority trial, PACE-B,23 reported acute toxicity and efficacy findings with ultra-hypofractionation versus conventionally fractionated or moderately hypofractionated radiotherapy in 874 patients with low- to intermediate-risk localized prostate cancer.24 Notably, this study showed that ultra-hypofractionation increased neither GI nor GU acute toxicity. More specifically, grade ≥2 severe toxic GI AEs occurred in 12% of patients in the conventionally fractionated or moderately hypofractionated radiotherapy group versus 10% of patients in the ultra-hypofractionation group (p=0.38), while grade ≥2 GU toxicity was reported in 27% versus 23% of patients, respectively (p=0.16). Efficacy was extremely high with 95% event-free survival at 5 years.
Overall, ultra-hypofractionated radiotherapy for localized prostate cancer demonstrates favorable early results, both in terms of efficacy and toxicity. It is very likely that this type of treatment will become the preferred treatment option within the next 5 years.
Elective irradiation of the lymphatic drainage pathways: a contentious issue
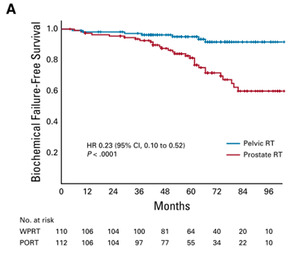
In patients with localized prostate cancer, two earlier randomized trials, NRG/RTOG 94136 and GETUG-01,5,25 assessed HT plus elective radiotherapy of the pelvic lymphatics; however, the results from these two studies were inconclusive. In detail, NRG/RTOG 9413, a 2 × 2 factorial study with hormonal sequencing as one stratification factor and radiation field as the other factor, showed that neoadjuvant HT plus whole pelvic radiotherapy (WPRT) improved progression-free survival compared with neoadjuvant HT plus prostate-only radiotherapy (PORT) and WPRT plus adjuvant HT in patients with intermediate-risk and high-risk localized prostate cancer.6 The major limitation of the trial was its lack of power to draw definite conclusions, as well as the unclear interaction of WPRT and HT-timing. On the other hand, the long-term data from the GETUG-01 study indicated that WPRT did not statistically improve either event-free survival or overall survival (OS) in this patient population.25
More recently, results were reported from the phase III, single-center, randomized controlled POP-RT trial, comparing elective WPRT to PORT in patients with high-risk prostate cancer.26 In this study, 224 patients underwent 1:1 randomization to receive either WPRT (68 Gy/25 fractions to the prostate, 50 Gy/25 fractions to pelvic nodes) or PORT (68 Gy/25 fractions to the prostate). The primary endpoint of the 5-year BFFS rate was 95.0% with WPRT versus 81.2% with PORT (unadjusted HR: 0.23 [95% CI: 0.10−0.52]; p<0.0001) (Figure 2). WPRT was also associated with higher 5-year disease-free survival rates at 89.5% versus 77.2% (HR: 0.40 [95% CI: 0.22−0.73]; p=0.002) and rates of distant metastasis-free survival (95.9% vs 89.2%; HR: 0.35 [95% CI: 0.15−0.82]; p=0.01), but 5-year OS rates were only numerically higher (92.5% vs 90.8%; HR: 0.92 [95% CI: 0.41−2.05]; p=0.83). Notably, there was no significant difference in acute GU and GI toxicities between the two treatment arms.
The current guidelines leave this issue open. For example, S3 Guidelines for prostate cancer disclose that the value of irradiation of the pelvic lymphatic drainage pathways in addition to prostate irradiation in patients with localized prostate carcinoma of intermediate and high intermediate-risk and high-risk profiles has not been clarified.7 Furthermore, the NCCN guidelines recommend that elective nodal radiation can be considered if additional risk assessments suggest aggressive tumor behavior in patients with unfavorable intermediate risk.8 Androgen deprivation therapy (ADT) should be used unless additional risk assessments suggest less-aggressive tumor behavior or if medically contradicted. In patients with high and very high risk, elective nodal radiation can be considered.
Insights into side effects of surgery and radiotherapy
An accurate assessment of the safety of radiotherapy and surgery is crucial for the appropriate management. Clinical trials reporting on safety often use AEs assessment tools performed by physicians, which represents a risk of missing the patient’s voice.27–29 In fact, data suggest that physicians’ reports of patients’ AEs may be unreliable.30 As a result, patient-reported outcomes (PROs) have emerged as an important tool to measure a patient’s perception of their symptoms and are increasingly used in clinical trials.
Comprehensive PROs were reported in the Prostate Testing for Cancer and Treatment (ProtecT) trial, which compared outcomes with active monitoring, radical prostatectomy and external-beam radiotherapy in 1,643 men with localized prostate cancer.9 Briefly, regarding efficacy, there was no significant difference in mortality (p=0.48) and the number of deaths from any cause (p=0.87) across the three groups of patients with mainly low-risk prostate cancer. In contrast, the active monitoring group was associated with higher rates of disease progression (p<0.001) and metastases. Further data showed that PROs in urinary, bowel, sexual function and quality of life differed among the three treatment groups, based on completed questionnaires before diagnosis, at 6 and 12 months after randomization and annually thereafter.10 Of the three therapies, prostatectomy had the greatest negative effect on urinary continence and sexual function and despite some recovery, these outcomes remained worse in this group throughout the trial. Radiotherapy was not associated with the worsening of urinary continence, while its negative impact on sexual function was greatest at 6 months, followed by recovery. Sexual and urinary function declined gradually in the active-monitoring group. Bowel function, urinary voiding and nocturia were worse in the radiotherapy group at 6 months but then somewhat recovered, except for the increasing frequency of bloody stools. No significant differences were observed among the groups in measures of anxiety, depression or general quality of life. Comparable results were reported in the 12-year update of ProtecT.31 Over 7 to 12 years, the highest proportion of patients in the prostatectomy group experienced urinary leakage requiring pads versus the active monitoring and radiotherapy groups (18−24% vs 9−11% and 3−8%). Erections sufficient for intercourse were reported in 18% versus 30% and 27% of patients, respectively, at 7 years; all converged to low levels of potency by year 12. Furthermore, at 12 years, nocturia occurred in 34% of patients receiving prostatectomy versus 48% of those receiving radiotherapy and 47% of those receiving active monitoring, while fecal leakage was reported in 12% versus 6% and 6% of patients, respectively. The declines in sexual and urinary function in active monitoring group are both gradual age-related, as well as due to opting for a definite treatment (prostatectomy or radiotherapy).
Another study, PACE A, aimed to determine if patients (n=123) achieved QoL improvement following SBRT (36.25 Gy/5 fractions in 1−2 weeks) compared with surgery.32 At 2 years, significantly fewer SBRT-treated patients reported the use of urinary pads (4.5% vs 46.9%; p<0.001). Similarly, patients receiving SBRT versus surgery had improved sexual bother subdomain score at 2 years (57.7 vs 29.3; p<0.001), while there was no difference in urinary subdomain score (85.5 vs 80.5; p=0.29). In contrast, the bowel subdomain score was significantly worse with SBRT (mean, 88.4 vs 97.3; p<0.001), with 15.6% and 0% of patients, respectively, reporting small/moderate problems with bowel symptoms (p=0.04). Grade ≥2 GU toxicity was observed in 9.3% of patients in the SBRT group and 9.5% of patients in the surgery group (p=0.97); there were no grade ≥2 GI AEs in either group.
Conclusions
-
Moderate hypofractionated radiotherapy is the standard for primary radiotherapy in patients with localized prostate cancer, with comparable efficacy and long-term tolerability to that of conventionally fractionated radiotherapy. Ultra-hypofractionated radiotherapy in prostate cancer appears very promising.23
-
Based on data from the randomized, phase III POP-RT trial, elective lymphatic drainage pathway irradiation can be recommended in patients with corresponding risk for subclinical lymph node involvement.
-
Radiotherapy and surgery are associated with a different spectrum of possible AEs. In general, incontinence and impotence mainly occur in patients receiving surgery, while bowel problems appear almost exclusively after radiotherapy.
Conflict of interest
The author declares that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author created and approved the final manuscript.