Introduction
Roughly 60% of all cancer patients are older than 65 years at diagnosis and many cancers are linked to aging, like breast, colorectal, pancreatic, prostate and lung cancers.1,2 Geriatric oncology faces unique challenges as physiological changes that occur with aging, such as reduced cardiovascular performance, decreased respiratory and renal function, as well as compromised nervous system, affect the tolerability of older patients to cancer therapy.3–5 Ongoing research aims to elucidate the factors associated with poor prognosis and high toxicities of treatment in this population. At the SIOG 2022 Annual Conference, with the theme “Celebrating resilience”, recent developments in the field of geriatric oncology were presented. In this article, we provide an overview of key relevant presentations.
The need for evidence-based data in older cancer patients
Although endocrine therapy (ET) combined with a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor is the standard of care for hormone receptor (HR)-positive advanced breast cancer, the prognostic impact of advanced age on treatment outcomes is not established. In a retrospective analysis, the safety and efficacy of palbociclib plus ET was compared in patients aged ≥65 years (n=31; median age: 72 years) and those aged <65 years (n=35; median age: 56 years).6 The incidence of grade ≥3 neutropenia was higher in the elderly compared with the younger group, with numerically higher rates of associated palbociclib dose reduction (p=0.309). The median progression-free survival (PFS) for first-line palbociclib plus an aromatase inhibitor (AI) was 26 months and 11 months for second-line palbociclib plus fulvestrant. No significant PFS differences were observed based on age (young vs old, HR: 0.51 [95% CI: 0.17–1.56]; p=0.24) and dose reduction (no vs yes, HR: 0.93 [95% CI: 0.34–2.55]; p=0.89). Overall, these results suggest that palbociclib was well-tolerated and effective in the geriatric population.
In the triple-negative breast cancer (TNBC) setting, older patients have a greater risk for toxicities with neoadjuvant and adjuvant chemotherapy and a poorer prognosis for stage I−III disease due to comorbidities. A recent retrospective study assessed treatment outcomes with anthracycline-based and taxane-based chemotherapy in patients aged ≥65 years with stage I−III TNBC.7 A validated frailty index (FI) was used to calculate the comorbidity score. The study included 79 patients, with a mean age of 71 years and a mean FI of 0.13; the majority of patients (83.5%) were fit and 16.5% were pre-frail; no patients were classified as frail. Overall, 46% of patients received an anthracycline-containing regimen, with increased use in later-stage disease (stage I/II/III: 18%/52%/76%). Anthracycline-based compared with taxane-based chemotherapy showed a nonsignificant trend for higher treatment toxicity, defined as dose reduction (28% vs 24%), unplanned hospitalization (28% vs 12%), grade 4 hematologic toxicity (28% vs 14%) and grade 3−4 non-hematologic toxicity (33% vs 17%). The median overall survival (OS) was 43.5 months with anthracyclines and 63.0 months with taxanes (p=0.212). In summary, fit older patients showed better tolerability and survival with a taxane-based regimen, although results were not statistically significant and potentially confounded by the chemotherapy choice in earlier stages.
Another study presented at the SIOG 2022 investigated the effect of chemotherapy on the immune system of older patients with breast cancer.8 This longitudinal study included women aged ≥70 years with early-stage disease who received either adjuvant chemotherapy (n=39) or aromatase inhibitors only (n=32). Analysis of 6 genes associated with immunosenescence genes was performed on peripheral blood mononuclear cells (PBMCs) collected before treatment initiation and at 3 and 12 months after initiation of adjuvant therapy. Results showed that chemotherapy induced a transient reduction but a long-term increase in the expression of immune-related genes involved in T-cell activation and cell migration, suggesting a potential stimulation of immunity in older patients with breast cancer.
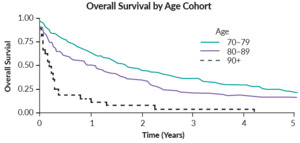
The challenge of treating older patients with gynecological cancers was well illustrated in a Canadian retrospective study, which enrolled 440 women with ovarian cancer who were aged ≥70 years (median, 78.4 years) at the time of diagnosis.9 More than half of them had an International Federation of Gynecology and Obstetrics (FIGO) stage III/IV disease (56%) and 59% received no chemotherapy. Of patients who were treated with chemotherapy, 20% received ≤2 cycles and 21% required dose reduction due to toxicity. OS analysis by age showed that OS decreased with increasing age, with patients ≥90 years old having the worst prognosis (Figure 1). A multivariable analysis further indicated that advanced stage, treatment modality and advanced stage are associated with poor prognosis (all p<0.001). The risk factors associated with intolerance and treatment discontinuation should be investigated in this population.
Novel treatments have improved survival in patients with metastatic colorectal cancer (mCRC), however, older patients have largely been excluded from pivotal trials and data are therefore scarce. A population-based study using data from the Netherlands Cancer Registry between 2005 and 2020 investigated the treatment patterns and survival in 22,192 patients aged ≥70 years with synchronous mCRC.10,11 OS was assessed separately for those diagnosed from 2014 onwards due to the introduction of a CRC screening program. From 2015 to 2020, chemotherapy use decreased (34% in 2020), while the use of targeted therapy given together with first-line chemotherapy remained generally stable at ~20% in that period. Over time, more patients received best supportive care (46% in 2020). OS of patients with metastatic colon and rectal cancer improved until 2014. From 2014 onwards, OS outcomes were worse in patients with colon cancer (HR: 1.04 [95% CI: 1.01–1.05]), while they remained unchanged in those with rectal cancer (HR: 1.00 [95% CI: 0.98–1.03]). As these trends may reflect the undertreatment of older frail patients due to low chemotherapy use, tailored chemotherapy such as upfront dose reductions should be investigated in this population.
The benefit of geriatric and patient-reported assessment tools in clinical decision-making
Also the elderly with lung cancer are underrepresented in randomized clinical trials, although approximately half of them are ≥70 years old. In a prospective study presented at SIOG 2022, safety and survival outcomes of 52 patients aged ≥70 years with stage III unresectable non-small cell lung cancer (NSCLC) were investigated according to geriatric assessment (GA).12
A GA based on the Balducci criteria was performed before the initiation of treatment but treatment was independent of the result. At baseline, 41% of patients were classified as fit, 24% as vulnerable and 35% as frail. In total, 73% of patients were considered fit for chemotherapy, 66% received chemotherapy with dose reduction and 78% were treated with radiotherapy (mean, 64 Gy); 67% received both chemo- and radiotherapy (57% concomitant, 43% sequential). During follow-up, 17% of patients died, mainly due to progressive disease (61%). Overall, symptomatic patients and those with exclusive chemo- or radiotherapy had inferior OS outcomes. Frail patients showed a trend towards worse OS (median, 11 months vs 30 months; HR: 2.34; p=0.07) (Figure 2) and higher toxicity (40% vs 18%; p=0.21). Thus, GA is a useful method to identify frail patients who might benefit from a tailored treatment approach.
In another prospective study, the prognostic value of 12 GA domains was analyzed in 123 elderly patients (median age: 79.9 years) with stage I−III early BC.13 GA was performed prior to adjuvant treatment. Stage I, II and III disease was diagnosed in 36%, 42% and 23% of patients, respectively, and the subtype was identified as luminal A, luminal B and TNBC in 35%, 57% and 8% of patients. The 3-year rate of deterioration-free survival (DFS), defined as the time from diagnosis to loss follow-up, was 76% (95% CI: 58–85%), with all GA domains except for social status showing significant association with DFS (Table 1). The data support the integration of GA in clinical practice for patients with early BC.
As malnutrition has been associated with adverse surgical outcomes, identifying patients at nutritional risk prior to surgery is essential. A study by Refaeli et al. (2022) evaluated the nutritional status of patients ≥65 years with gastrointestinal (GI) cancers prior to abdominal surgery using Mini Nutritional Assessment (MNA) as part of a comprehensive GA.14 Among 75 patients (mean age: 78.7 years), colon cancer was most common (45%), followed by cancer of the upper GI (esophagus/gastric) (31%) and hepatobiliary cancer (21%). Weight loss of >10% and 5−10% were each observed in 23% of patients, with the highest rate among patients with upper GI cancers. Overall, malnutrition was found in 23% of patients and 37% were at risk of malnutrition (upper GI: 47% and 37%, colon: 9% and 35%, hepatobiliary: 8% and 50%, respectively). This study emphasized the need for preoperative nutritional assessment in the elderly, with further studies investigating the impact of nutritional interventions on surgical outcomes.
Patient-reported outcome measurements (PROMs) are important tools for assessing the quality of life (QoL) in geriatric care. In a single-center study, real-world data were assessed from a PROM program in older patients with lymphoma receiving intravenous chemotherapy.15 The primary endpoint of this analysis was the adherence of older patients to the program, as well as its potential benefit in reducing toxicity and unscheduled visits. Of 138 enrolled patients, 48 (34.8%) were ≥70 years old and 15 of those (31.3%) submitted PROMs reports. No differences in adherence due to age were observed and older patients did not report more adverse events (AEs) than younger ones, except for mood-related symptoms (46.7% vs 3.4%; p<0.01). PROMs were also associated with fewer emergency room visits (60.6% vs 47.9%).
Factors associated with treatment outcomes
Data showed that infectious diseases including COVID-19 may contribute to cancer-related deaths, which was further corroborated in a recent study assessing the factors associated with mortality in older cancer patients with SARS-CoV-2 in Brazil (n=604).16 The global mortality rate was 74%. Based on Shapley additive explanations (SHAP) analysis, vaccination was the most influential factor and the only protective factor in the model, while other features like age and male sex were risk factors associated with death.
Computed tomography (CT)-based measurements of skeletal muscle cross-sectional area and density reflect physical function. In a retrospective study, the associations of skeletal muscle quality using a CT-based muscle index with GA and OS were assessed in older patients with cancer.17 The study enrolled 277 patients aged ≥60 years, most frequently with lung (45.1%) and GI (38.9%) cancer; 53.7% of patients had metastatic disease. The evaluation of the skeletal muscle index was performed by drawing a region of interest on the left and right para-spinal muscle at the L3 vertebral level, one section above and below. Patients aged ≥75 years and women had a lower muscle index, which was associated with impaired basic and instrumental activities of daily living and frailty. Patients with poor muscle quality showed inferior OS (p=0.021).
An increase in inflammatory marker levels has been associated with advanced age, cancer risk, functional decline and minoritized racial groups. The relationship between interleukin (IL)-6, a pro-inflammatory cytokine, and functional capacity among older cancer patients, including the effect of racial status, have been assessed in an analysis using data from the Health, Aging and Body Composition (Health ABC) study (n=3,075; follow-up: up to 16 years) that evaluated risk factors for functional impairment in the elderly (age: 70–79 years).18,19 In total, 179 patients with newly diagnosed cancer and available IL-6 measures within 2 years of diagnosis and ≥3 longitudinal functional measures post-diagnosis were included. The assessed measures were the self-reported ability to walk a quarter of a mile (functional status [FS], 0-unable, 6-very easy) and 20-meter gait speed (GS). The longitudinal trajectory model identified three clusters for walking performance based on the FS parameter: high stable, decline and low stable. Higher levels of IL-6 were found in the low-stable versus high-stable groups (odds ratio [OR]: 2.34 [95% CI: 1.04–5.24]). In terms of GS, the model identified two clusters: resilient and decline, with higher IL-6 levels reported in the decline versus resilient groups (OR: 4.51 [95% CI: 1.55–17.25]). Both associations were stronger in white compared with black patients (36% of the cohort).
Conflict of interest
The author received honoraria for consultancy from GSK, Roche, Novartis, Exact Sciences, Pfizer, Stemline, AbbVie and ASC Oncology. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_bas.png)
_domains_with_deterioration-free_survival_(dfs.png)

_bas.png)
_domains_with_deterioration-free_survival_(dfs.png)