Prostate-specific membrane antigen (PSMA)
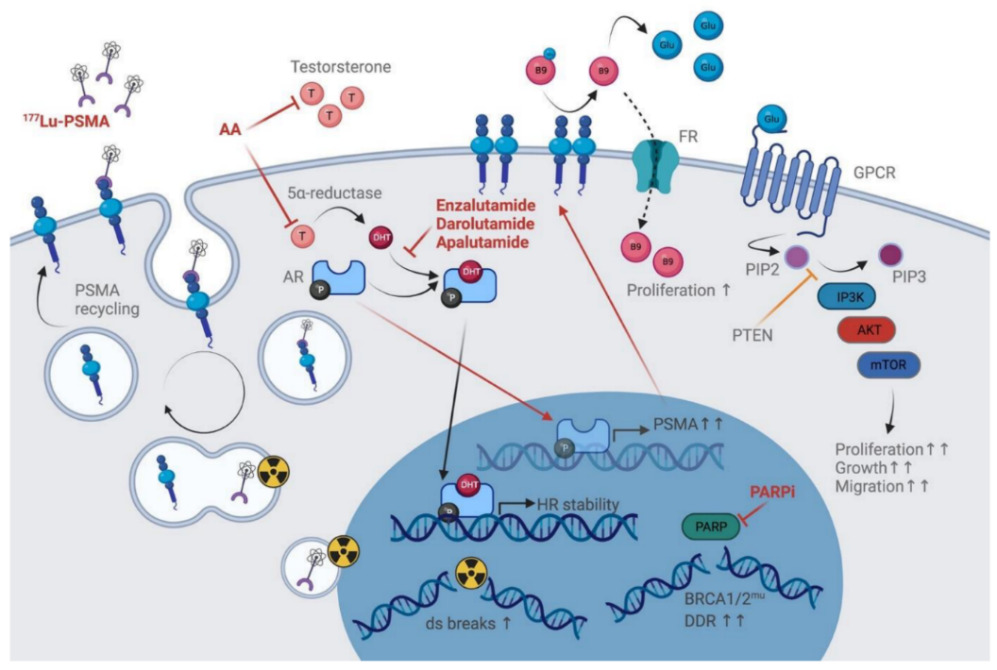
Prostate-specific membrane antigen (PSMA), encoded by the folate hydrolase 1 (FOLH1) gene, is a type II transmembrane-bound glycoprotein (Figure 1, left) with two well-defined enzymatic functions depending on its location within the body: folate-hydrolyzing activity in the small intestine and N-acetyl aspartyl glutamate (NAAG)-hydrolyzing activity in the nervous system.1,2 PSMA is detected in the prostate epithelium, small intestine, renal tubules, celiac ganglia and salivary glands.3,4 It is highly expressed in patients with prostate cancer (PCa) (100−1,000-fold compared with other tissues), particularly in poorly differentiated primary tumors and metastatic lesions.3–8 This overexpression allows for an increased folate uptake that is crucial for one-carbon metabolism and confers a survival advantage in a folate-deprived environment (Figure 1, right).2 In PCa, dysregulation of the folate metabolic pathway has been shown to correlate with a shorter time to biochemical recurrence (BCR). In addition, PSMA releases free glutamate from its ligands, which activates the PI3K/Akt/mTOR pathway and is implicated in PCa growth.9 Notably, patients with high-grade tumors present with high serum glutamate levels.
Given the PSMA function in PCa, its regulation has been widely studied.2 Androgens are known to downregulate PSMA, a process potentially mediated by the common TMPRSS2-ERG fusion (identified in ~50% of cases).2,6,10 In addition, DNA damage repair (DDR) aberrations have been associated with increased PSMA expression.8
The prognostic value of PSMA is well established. High PSMA expression significantly increases the likelihood of BCR.11–13 Hupe et al. (2018) demonstrated 5-year recurrence-free survival rates of 88.2%, 74.2%, 67.7% and 26.8% for patients demonstrating no, low, medium and high PSMA expression on biopsy, respectively.11 In metastatic castration-resistant prostate cancer (mCRPC), membranous (m) PSMA expression level has been negatively associated with a higher Gleason grade and worse overall survival (OS).8 The study further demonstrated higher levels in patients with mCRPC versus castration-sensitive prostate cancer (CSPC) (median H-score: 55.0 vs 17.5), but with poor concordance between matched samples. Importantly, PSMA expression shows marked inter- and intratumor heterogeneity.8,14 Paschalis et al. (2019) reported that 42% of CSPC and 27% of mCRPC biopsies had no detectable mPSMA (H-score <10) and 100% mPSMA-expressing CSPC and 84% of mCRPC biopsies showed marked intratumor heterogeneity of mPSMA expression, with foci containing no detectable PSMA.8
As a result of PSMA overexpression in PCa and its limited expression in healthy tissues and benign prostate tumors,2,4–6 a variety of PSMA low-molecular-weight inhibitors have been developed, with the potential to be used for treatment strategies that combine therapeutics with diagnostics, collectively termed theragnostics.15 By binding to PSMA, these small molecules emit radiation, allowing the visualization using positron emission tomography (PET).16 Radionuclide-labeled monoclonal antibodies have displayed several drawbacks, among which the slow clearance from non-target tissues.17,18 Thus, small molecule PSMA-peptide inhibitors are now the mainstay of current PSMA imaging and treatment modalities.19,20 These include 68Gallium (Ga)- and 18Fluorine (18F)-radiolabeled PSMA (e.g., radiotracers 68Ga-PSMA-11, 18F-DCFPyL and, more recently, 18F-PSMA-1007 that combine the high specificity of the antibody/minibody tracers with a superior biodistribution.20–23 Evidence from the literature suggests that these radiotracers can detect PCa relapses and metastases with high contrast by binding to the extracellular domain of PSMA, followed by internalization.20,21
Diagnostic applications of PSMA hybrid imaging
Staging
The role of 68Ga-PSMA PET/computed tomography (CT) imaging is being evaluated for both primary staging and restaging of PCa. The landmark multicenter, randomized phase III proPSMA study showed that 68Ga-PSMA-11 PET/CT (n=150) has superior accuracy with lower radiation exposure compared with conventional CT and bone scan imaging (n=152) for staging high-risk prostate cancer before curative surgery or radiotherapy.24 This prospective study changed treatment practice with almost immediate effect, demonstrating that PSMA PET/CT outperforms conventional imaging inaccuracy (92% vs 65%; p<0.0001), sensitivity (85% vs 38%) and specificity (98% vs 91%), respectively. Moreover, it showed that PSMA PET/CT was more sensitive and specific than conventional imaging for nodal and distant metastatic disease in patients with high- and very high-risk PCa. Management change with a high or medium effect was provided for 28% of patients who underwent first-line PSMA PET/CT versus 15% of patients who underwent conventional imaging (p=0.008). Among patients who crossed over to second-line imaging, PSMA PET/CT had a high or medium effect in 27% of men versus 5% with conventional imaging.
Similar results were demonstrated in a retrospective analysis of 116 intermediate or high-risk PCa patients.25 In this study, the application of 68Ga-PSMA-11 PET/CT, compared with clinical staging and conventional imaging, resulted in additional/unknown information in approximately 36% of patients (42/116), while 27% of patients (32/116) would most likely have received a change in their management, either to a different therapy modality or adjusted treatment details. Compared with histopathology, 68Ga-PSMA PET showed an improved detection accuracy for pelvic nodal metastases prior to radical resection and pelvic lymph node dissection in men with intermediate- to high-risk disease in a prospective phase III trial (n=277), with a sensitivity, specificity, positive predictive value (PPV) and negative predictive value of 0.40, 0.95, 0.75 and 0.81, respectively.26 These results suggest 68Ga-PSMA PET at initial staging may guide patient care, although it should not be a substitute for lymph node dissection.
Biochemical recurrence
Despite local definitive primary therapy (radical prostatectomy or radiotherapy), up to 40% of men with intermediate- or high-risk PCa will develop recurrent disease.27 Early salvage radiotherapy improves outcomes in PCa patients who develop BCR after radical prostatectomy.28,29 Imaging can provide valuable data in this setting, particularly allowing the identification of localized recurrence and guiding specific treatment strategies.30,31 However, many PCa patients with BCR show no evidence of metastasis with modern imaging techniques due to low sensitivity, especially at low serum prostate-specific antigen (PSA) levels (e.g., <1.0–1.5 ng/mL).30 PSMA PET/CT has greatly increased the diagnostic accuracy of BCR PCa by detecting atypical metastatic lesions and lesions without any morphologic changes and at unusual sites with high specificity, which are missed with conventional imaging methods.30 In a prospective study of a small cohort of patients with BCR (n=70) and low PSA levels (<1.0 ng/mL) considered for salvage radiotherapy, PSMA-positive lesions using 68Ga-PSMA PET/CT were detected in 54% of patients.32 Notably, application led to a major management change in almost 30% of patients based on the results of the 68Ga-PSMA PET/CT study. A retrospective study by Eiber et al. (2015) in patients with BCR following radical prostatectomy (n=248) further demonstrated that imaging with PSMA PET/CT using the 68Ga-PSMA ligand significantly increased the detection rate at low serum PSA levels.33 Recurrence detection rates were 57.9%, 72.7% and 93.0% at PSA levels 0.2 to <0.5 ng/mL, 0.5 to <1 ng/mL and 1 to <2 ng/mL, respectively. Improved sensitivity with rising PSA levels has been seen across studies.34–36 In the largest prospective study in patients with BCR following initial therapy with curative intent (n=2,005), PSMA-positive lesions were reported in 50.5% for PSA values between 0.25 ng/mL and <0.5 ng/mL, 69.2% for PSA values between 0.5 ng/mL and <1.0 ng/mL and 78.1% for PSA values between 1.0 ng/mL and <2.0 ng/mL, with the largest differences in sensitivity observed for prostatectomy patients.36 The overall scan positivity rates were 66.6% following radical surgery, 81.8% following post-operative radiotherapy (RT) and 95.4% following definitive RT; for pelvic nodal disease, these rates were 42.7%, 50.8% and 38.8%, respectively. Furthermore, the PPV through histopathological verification was 0.82 (146/179) for all assessed samples and 0.72 (34/47) for pelvic lymph node samples. Table 1 summarizes the detection rates of 68Ga-PSMA PET across PSA levels, including data on [18F]Choline PET/CT for comparison. The increased detection of PSMA-positive lesions outside the prostate bed in BCR PCa has resulted in the adoption of PSMA-guided metastases-directed treatment (MDT) in this setting. In a retrospective study, radiotherapy based on PSMA PET in oligorecurrent PCa resulted in a 2-year BCR-free survival (bRFS) rate of 53% (n=190), with 93% of patients not having initiated androgen deprivation therapy (ADT) after 1 year.37 Notably, ADT concurrent with MDT prolonged bRFS compared with MDT alone (n=115, HR: 0.28 [95% CI: 0.16–0.51; p<0.0001), with a 2-year rate of 78% and significantly better results in patients receiving long-term versus short-term ADT.
In addition to 68Ga-PSMA-11, many alternative radioligands have become available, including, but not limited to, 18F-labeled PSMA-radiotracers such as 18F-DCFPyL or 18F-PSMA-1007. 18F-labeled PSMA-radiotracers have different advantages and disadvantages compared with 68Ga-labelled ligands (Table 2). In the setting of BCR, where 18F-PSMA-1007 may have an added advantage, a pilot study by Giesel et al. (2018) found high detection rates (75%), comparable with 68Ga-PSMA ligands, with very low radiotracer accumulation in the urinary system.39 More recently, in a head-to-head comparison of 18F-PSMA-1007 and 68Ga-PSMA-11 PET/CT, similar results were reported for the two radiotracers in terms of primary staging, BCR and evaluation of PCa patients with metastatic disease.40 Notably, 18F-PSMA-1007 PET/CT detected a few additional lesions compared with 68Ga-PSMA. It is important to consider that local unspecific bone uptake on 18F-PSMA-1007 PET seems a frequent finding, possibly hindering the detection of bone metastases and leading to PCa overstaging.41 A clinical evaluation of 18F-PSMA-1007 by Grünig et al. (2021) showed unspecific bone uptake in 51.4% of patients, of which 44.7% were considered clinically relevant since these false-positive lesions influenced the treatment approach.41
PSMA PET is increasingly used to select the optimal treatment strategy in patients with BCR, resulting in management changes, and together with genomic testing should lay the ground for precision medicine.34,42,43 Accurate and reproducible quantification and interpretation of the PSMA PET/CT scans are crucial for effective diagnosis and approaches like applying artificial intelligence to medical imaging are being explored to automate and standardize this complex process.44
Role of PSMA PET in non-metastatic castration-resistant prostate cancer
Accurate evaluation of the extent of PCa spreading is very important in guiding suitable treatment at all disease stages. In a retrospective study that included 200 patients with non-metastatic (nm)CRPC, PSMA PET detected nearly all localized advanced disease (M0) and 55% of distant metastases (M1) in patients previously diagnosed with nmCRPC.45 However, whether earlier progression events detected by PSMA PET are clinically meaningful and will translate into worse clinical outcomes remains unknown.46 Furthermore, the benefits of PSMA PET imaging for changing the treatment approach are not yet proven. Clinical trials correlating PSMA PET staging and data on outcomes such as metastasis-free survival (MFS) and OS are needed to define the clinical utility of PSMA PET better.46 For men who are M0 on conventional imaging but M1 on PSMA PET staging, the phase II DECREASE trial aimed to investigate whether the addition of consolidation radiotherapy in addition to anti-androgen therapy in chemotherapy-naïve mCRPC patients will improve clinical outcomes of patients compared with those receiving anti-androgen therapy alone.47 The results of this trial will help inform whether disease stage (M0 or M1) as determined by PSMA PET/CT can be used to guide treatment decisions instead of conventional imaging.46
PSMA-targeted radioligand therapy for patients with mCRPC
Current standard treatments alter PSMA expression, a topic extensively reviewed elsewhere.48 Androgen blockade briefly increases PSMA expression (Figure 2), with conflicting findings in CSPC and evidence for negative PSMA regulation by prolonged ADT.2,48–54 While the reduced tracer uptake might be a result of therapy-related tumor shrinkage as opposed to lower PSMA expression per cell, this heterogenous effect of ADT has led to some controversy for treatment planning, specifically the possibility of ADT suspension for increasing PSMA PET sensitivity and improving patient selection for radioligand therapy.
Lutetium-177 (177Lu)-PSMA-617 is a small molecule that enables highly targeted delivery of β-radiation to PSMA-expressing cells. It is the first targeted radioligand therapy approved for the treatment of patients with PSMA-positive mCRPC by the Food and Drug Administration (FDA)56 and, very recently, by the European Medicines Agency (EMA)57 and the Swissmedic58 due to the results from the open-label, phase III VISION trial.59 VISION was designed based on positive results from two phase II LuPSMA and TheraP trials (Table 3).
The unblinded, randomized phase II TheraP trial assessed 177Lu-PSMA-617 in patients with mCRPC for whom cabazitaxel was considered the next appropriate standard treatment.62 Patients were assigned to receive either 177Lu-PSMA-617 (n=99) (6.0–8.5 GBq intravenously every 6 weeks for up to 6 cycles) or cabazitaxel (n=101) (20 mg/m2 intravenously every 3 weeks for up to 10 cycles) and evaluated for PSA decline ≥50%. In the treated population, 177Lu-PSMA-617 was associated with significantly improved rates of PSA responses versus cabazitaxel (66% vs 44%; p=0.0016). The incidence of grade 3-4 adverse events (AEs) was 33% in the 177Lu-PSMA-617 group and 53% in the cabazitaxel group.
After a median follow-up of 3 years, the study did not meet its secondary endpoint of OS in the intention-to-treat population (HR: 0.97; p=0.99) (Figure 3A).63 The restricted mean survival time (RMST) was 19.1 months with 177Lu-PSMA-617 and 19.6 months with cabazitaxel. However, 177Lu-PSMA-617 significantly delayed progression versus cabazitaxel (HR: 0.62 [95% CI: 0.45−0.85]; p=0.0028) (Figure 3B), with RMST of 7.1 months versus 5.0 months, respectively. In this follow-up analysis, no additional safety signals were identified, suggesting a more favorable profile of 177Lu-PSMA-617. Altogether, these data support 177Lu-PSMA-617 over cabazitaxel for the treatment of patients with PSMA-positive, progressive mCRPC after taxane-based chemotherapy and androgen receptor-pathway inhibitor (ARPI).
In the randomized VISION trial, 177Lu-PSMA-617 was investigated in patients with mCRPC previously treated with ≥1 ARPI and 1−2 taxane regimens and who had PSMA-positive 68Ga-PSMA-11 PET/CT scans.59 A total of 831 patients were randomized 2:1 to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 4–6 cycles) plus protocol-permitted standard of care (SoC) or SoC alone. It should be noted that the interpretation of results are limited by the choice of SoC therapies, which was not reflective of modern clinical practice. At a median follow-up of 20.9 months, 177Lu-PSMA-617 plus SoC versus SoC alone significantly improved imaging-based progression-free survival (PFS) (median: 8.7 months vs 3.4 months; HR: 0.40 [99.2% CI: 0.29–0.57]; p<0.001) and OS (median: 15.3 months vs 11.3 months; HR: 0.62 [95% CI: 0.52–0.74]; p<0.001) in this patient population (Figure 4A and 4B). In addition, more favorable health-related quality of life and pain outcomes were seen in the investigational versus control arm, despite higher rates of grade ≥3 AEs (52.7% vs 38.0%).
An exploratory analysis of VISION further evaluated radiographic PFS and OS according to prior and concomitant cancer-directed treatments, including the number of prior ARPIs, taxane regimens, non-taxane regimens and immunotherapies; prior treatment with bone health agents (BHAs), 223 radium (Ra) and poly (ADP-ribose) polymerase (PARP) inhibitors; and concurrent ARPIs, radiation therapy and BHAs.64 The clinical benefits of 177Lu-PSMA-617 were consistent across subgroups regardless of prior treatment or SoC, with >40% reduction in risk of death observed in patients who had received ≥2 prior ARPIs, 1 taxane regimen, prior BHAs and no immunotherapy, as well as ARPIs and BHAs as part of SoC.
Notably, patients showing diffuse bone involvement on a “superscan” by bone scintigraphy at baseline were excluded from VISION.65 However, results from a multicenter retrospective analysis indicated antitumor activity of 177Lu-PSMA also in this patient population (n=43) despite the high tumor burden (median baseline PSA: 1,000 ng/mL), with a PSA decline ≥50% at 12 weeks of 58% and a median OS of 11.6 months.
Predictive factors and tools for radioligand therapy with 177Lu-PSMA-617
Patients with mCRPC who are candidates for 177Lu-PSMA-617 therapy are selected based on PSMA PET positivity, high tumor expression and lack of discordant fluorodeoxyglucose (FDG)/PSMA findings.2,59,61,62 Indeed, a recent post hoc analysis of VISION supported the use of quantitative PSMA PET imaging as a prognostic tool by demonstrating a strong association between higher whole-body mean standardized uptake value (SUVmean) and improved outcomes.66 Patients in the highest versus lowest SUVmean quartile had a median radiographic PFS of 14.1 months versus 5.8 months and a median OS of 21.4 months versus 14.5 months (SoC alone, 3.4 months and 11.3 months, respectively). Notably, patients deemed ineligible for 177Lu-PSMA-617 therapy due to low PSMA expression or discordant FDG-positive and PSMA-negative disease (n=16) were reported to have a median OS of only 2.5 months.67 In addition, a study exploring the use of dual-tracer (68Ga-PSMA and 18F-FDG) PET/CT in nmCRPC identified discordant disease in patients with an early PSA progression during castration, thus its prognostic value deserves further evaluation.68
Prognostic nomograms have also been developed to help the clinical decision-making for men with mCRPC who are eligible for 177Lu-PSMA therapy.69 A model incorporating traditional predictive characteristics such as time since diagnosis, history of chemotherapy and hemoglobin status with variables relevant in this patient population (e.g., SUVmean, number and site of metastatic lesions) stratified patients into low-risk versus high-risk groups, with a median OS in the complete set of 19.9 months versus 8.2 months (p<0.0001). Furthermore, patients with a higher PSMA expression had more favorable outcomes (OS, PSA-PFS and PSA decline ≥50%), whereas those with bone involvement were less likely to benefit from 177Lu-PSMA therapy.
Finally, the optimal use of PSMA as a predictive biomarker requires an accurate characterization of its expression, a better understanding of its regulation, as well as modulation by therapies.2 For example, tumors harboring defective DDR show higher mPSMA expression,8 however patients with versus without DDR alterations were not more responsive to PSMA-targeted radioligand therapy (PFS HR: 1.14; OS HR: 1.40; p>0.1 for both).70
225Ac-PSMA-617: Alpha therapy for mCRPC
225Actinium (Ac)-PSMA-617 is an alpha particle emitter labeled PSMA agent that has shown remarkable therapeutic efficacy in heavily pre-treated mCRPC patients. The first indicators of efficacy for 225Ac-PSMA-617 in mCRPC were demonstrated in a retrospective study on 40 patients.71 Of the 38 patients who survived at least 8 weeks post-treatment, 24 (63%) had a PSA decline >50% and 33 (87%) had any PSA response. The median duration of tumor control was 9.0 months. Furthermore, a pilot study showed that 82% of patients (14/17) achieved PSA decline ≥90%, including 7 patients with undetectable serum PSA following 2 or 3 cycles of therapy.72 In a follow-up retrospective study investigating 225Ac-PSMA-617 in a group of 73 patients, the rate of >50% PSA decline was 70% (any PSA decline: 82%), with a median PFS of 15.2 months and the median OS of 18 months.73
The activity of 225Ac-PSMA-617 was also assessed in a prospective study on 28 patients with mCRPC who either were either refractory or naïve to 177Lu-PSMA-617.74 Eight weeks after the first cycle of 225Ac-PSMA-617 therapy, >50% decline in PSA was observed in 25% of patients at the initial follow-up and 39% of patients the end of the follow-up. The median PFS was 12 months and the median OS was 17 months.
In a recent series of 53 patients treated with 225Ac-PSMA-617 administered following ADT, an impressive rate of PSA decline >50% at 91% was reported, with 96% of patients achieving any decline in PSA.75 A multivariate analysis further demonstrated that PSA decline of >50% might be predictive of PFS and OS in this clinical setting. At a median follow-up of 55 months, the median OS was estimated at 9 months for patients with a PSA decline <50% and not reached for those with PSA decline >50%.
In addition to 225Ac, other alpha particle emitter-labeled PSMA agents have been investigated so far, including 213Bi, 211At and 227Th.76–78
Other PSMA-directed therapies
Bispecific T-cell engagers (BiTEs) and chimeric antigen receptor (CAR) T-cell therapies have shown remarkable safety and efficacy in patients with hematological malignancies and are now being investigated in solid tumors like PCa using PSMA as a target. Among these, pasotuxizumab (also known as AMG 212 or BAY 2010112), a 55 kDa PSMA/CD3 BiTE, as monotherapy was safe and efficacious in CRPC patients in a first-in-human clinical trial, providing evidence of antitumor activity of BiTE monotherapy in solid tumors.79 Furthermore, AMG 160, a half-life extended (HLE) PSMA/CD3 BiTE, demonstrated strong antitumor activity in vitro and in mCRPC xenograft models, including potential synergy with enzalutamide or immune checkpoint blockade, as well as compatibility with 68Ga-PSMA-11.80 Based on these data, it is currently being evaluated in heavily pretreated mCRPC patients who were refractory to prior novel hormonal therapy and 1–2 taxane regimens and evidence of progressive disease.81
Encouraging data were also reported from early-phase studies on CAR T-cell therapies in prostate cancer. Results from a first-in-human phase I trial in CRPC-directed CAR T cells armored with a dominant-negative transforming growth factor (TGF)-β receptor showed that 5/13 patients developed grade ≥2 cytokine release syndrome (CRS) and >98% PSA reduction.82 This study suggested that clinical application of TGF-β-resistant CAR T cells is possible and generally safe for CRPC patients. Another therapy with genetically modified autologous T cells, CART-PSMA-TGFβRDN, were assessed in patients with mCRPC in the multi-center, open-label, phase I CART-PSMA-02 study.83 Initial findings reveal the antitumor activity of the therapy, however due to severe immune-mediated toxicities, without clearly understood mechanisms, the study was closed for further enrollment.84
PSMA antibody-drug conjugate (ADC) is an anti-PSMA monoclonal antibody conjugated to monomethylauristatin E, which binds to PSMA-positive cells and induces cytotoxicity.85 Results of the first-in-human, dose-escalation study of a PSMA ADC in a cohort of 52 men with mCRPC showed antitumor activity, with an acceptable safety profile.86 Based on these promising results, a phase II, open-label, single-arm trial was initiated.87 In the group of patients who progressed following abiraterone/enzalutamide therapy, PSMA ADC was associated with a PSA decline ≥50% in 14% of all treated and 21% of chemotherapy-naïve subjects. In addition, circulating tumor cells (CTCs) declines ≥50% were observed in 78% of all treated and 89% of chemotherapy-naïve patients.
In mCRPC patients after failure of abiraterone and/or enzalutamide and a taxane-based therapy, MEDI3726, another ADC-targeting PSMA, was evaluated in a phase I study.88 In the group of 33 patients who received MEDI3726, the composite response rate was 12.1% and the median composite duration of response was 3.8 months. At the data cut-off, the median PFS and the median OS were 3.6 and 9.8 months, respectively. However, clinical activity was mainly observed at higher doses with the worst toxicity profiles, but responses were not durable due to treatment-related adverse event (TRAE)-related treatment discontinuation.
Conclusions
-
PET/CT hybrid imaging of PSMA-positive lesions with the radiotracer 68Ga-PSMA-11 has heralded promising results for improving the diagnosis and treatment of PCa.
-
In primary PCa, PSMA PET/CT imaging has shown superior sensitivity and specificity for the detection of pelvic lymph nodes and distant metastases, resulting in subsequent clinical management changes.
-
In PCa patients with BCR, higher detection rates have been observed with PSMA PET/CT versus conventional imaging techniques, especially in post-radical prostatectomy patients with low serum PSA values.
-
The impact on decision-making for salvage radiation or treatment intensification remains to be defined.
-
PSMA-based imaging and radioligand therapy is a theragnostic approach and is presently applied for the treatment of patients with mCRPC.
-
Radioligand therapy with 177Lu-PSMA-617 is a promising treatment option for patients with mCRPC.
-
Several upcoming PSMA-directed therapies including BiTEs, ADCs and CAR-T cells are being investigated in randomized clinical trials for the management of patients with metastatic PCa.
Conflict of Interest
Alexander Meisel has provided a consulting, advisory, or speaker role for Amgen, Astellas, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Gerresheimer, GSK, Janssen, Merck, MSD, Novartis, Roche, Sanofi, Servier, Takeda and Vifor, has received research funding from Bayer, Sanofi, Gerresheimer (personal), and Merck & Cie (institutional), has intellectual property interests relating to Merck & Cie (not related to this report), has been paid to provide expert testimony for Sanofi, and has reported travel/accommodation expenses paid for by Amgen, Astellas, Boehringer Ingelheim, Janssen, Merck, Roche, Sanofi, and Servier. Alex Friedlaender received payments from Amgen, AstraZeneca, Roche, Astellas, Takeda, Bristol-Myers Squibb, Merck Sharpe Dohme, Pfizer, Merck, Novartis and Janssen. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way. Olivier Rager has declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author Contributions
All authors contributed to and approved the final manuscript.
_in_prostate_cancer_cells.png)

_overall_survival_(os)_and_b)_progression-free_survival_in_the_intention-to-treat_popula.png)
_imaging-based_progression-free_survival_and_b)_overall_survival.png)
_in_prostate_cancer_cells.png)
_overall_survival_(os)_and_b)_progression-free_survival_in_the_intention-to-treat_popula.png)
_imaging-based_progression-free_survival_and_b)_overall_survival.png)