Introduction
Plasma cell myeloma (PCM), or multiple myeloma, is characterized by the neoplastic proliferation of clonal plasma cells in the bone marrow.1 Symptomatic disease is characterized by end-organ damage and summarized by the calcium, renal function (creatinine), anemia (hemoglobin), and bone lesions, the so-called CRAB criteria (hypercalcemia, renal failure, anemia and bone lesions caused by activation of osteoclasts).2 The International Myeloma Working Group (IMWG) guidelines include these CRAB criteria to stratify the severity and prognosis of PCM.2,3
Induction chemotherapy followed by high-dose chemotherapy (HDCTx) and autologous stem cell transplantation (ASCT) remains the gold standard in treating eligible patients with PCM4,5 and proved superior over conventional treatment regarding response rate, event-free survival (EFS) and overall survival (OS) in several seminal clinical trials.5,6 The introduction of targeted therapies such as proteasome inhibitors (PI), immunomodulatory drugs (IMiDs) and, more recently, CD38-targeted antibodies further contributed to deeper remissions and improved survival outcomes in PCM patients.7–9 Treatment escalation via salvage ASCT after relapse or upfront tandem ASCT for patients with high-risk features represent further treatment options, and demonstrated benefit in progression-free survival (PFS) and OS in some studies.1,4,10–12 Despite significant treatment advances, PCM is still considered an incurable disease with virtually all patients eventually relapsing and only 10−15% of patients achieving or exceeding expected survival compared with the matched general population.
While the depth of remission is a major factor determining relapse dynamics, other factors including the cellular graft content that is retransfused following HDCTx might play a role in determining relapse patterns and dynamics. It has been demonstrated that apheresis products used for ASCT contain not only CD34+ peripheral blood stem cells (PBSCs) and hematopoietic progenitor cells but also tumor cells, potentially contributing to relapse when reinfused into patients.13–16 To reduce tumor cell contamination, various in vitro purging methods have been studied, for example, positive enrichment of hematopoietic stem cells by flow sorting using antibodies against CD34 and CD90.13,15,17–19 Yet it remains unclear whether in vitro purging of grafts will result in improved PFS and OS20 and the clinical impact of autografted tumor cells remains an unresolved concern.
In the current study, 204 PCM patients treated with ASCT at the University Hospital Zurich were retrospectively analyzed to evaluate patient- and treatment-related parameters and their associations with PFS and OS. The study particularly focused on the correlation between the number of reinfused cells and clinical outcomes as a surrogate for the potential role of concomitantly mobilized tumor cells in autologous grafts.
Materials and methods
This retrospective, single-center study included patients diagnosed with PCM who underwent ASCT between 2010 and 2014 in the Department of Medical Oncology and Hematology of the University Hospital Zurich. A subgroup of those patients received more than one ASCT. The clinical information system KISIM (CISTEC AG, Zurich, Switzerland) and the local transplant database were used for the data collection.
The remission status of patients at the time of ASCT and on day 100 post-ASCT was assessed according to the IMWG response criteria.3 Due to missing urine protein electrophoresis (PEP) and urine immunofixation (IF) results for nearly all patients these parameters were not included as response criteria, which is compliant with the clinical practice since serial serum free light chain (FLC) measurements are more suitable for response evaluation.21,22 Several other variables were investigated, including gender, age at the time of ASCT, type of induction therapy, number of HDCTx, total number of reinfused leukocytes and number of CD34+ cells, and the cause of death. Disease progression and relapse were evaluated using the IMWG criteria.3 Survival data were followed-up until August 2020.
The statistical analysis was performed using SPSS Version 26 (IBM Corp. Released 2019. Version 26. Armonk, NY, USA). Categorial variables are presented as counts and percentages while continuous variables are described by the mean, standard deviation (SD), range and median. Student’s t-test and Levene’s test for independent samples were used simultaneously to compare mean values between selected groups. A median test for independent samples was performed to compare medians of reinfused CD34+ cells between two groups of patients. Survival analyses were conducted using log-rank testing and the Kaplan-Meier estimator. P values <0.05 were considered statistically significant.
This study was approved by the cantonal ethics committee Zurich (BASEC-Nr. 2021-00157). The used methods were in accordance with the regulatory requirements of the human research act.
Results
Our study included 204 patients diagnosed with PCM and treated with HDCTx and ASCT. Of these, 62.7% were male. Out of all patients, 144 (70.6%) received one transplant, 26 (12.7%) received tandem (two scheduled ASCT due to high-risk cytogenetics) and 34 (16.7%) received ASCT as salvage therapy (one prespecified ASCT, followed by a second ASCT at relapse/progressive disease). Four patients (2.0%) received tandem ASCT plus a third salvage ASCT. These patients were included in either tandem or salvage groups, based on the type of their second transplant.
The mean age at the time of ASCT was 57.4 years (SD 8.6; range: 28.8−70.8; median: 58.4 years). Regarding induction therapy, 65.2% of patients received PI-based, 9.3% IMiD-based and 23.0% both PI- and IMiD-based regimens. Only five patients received an induction regimen containing neither PIs nor IMiDs. Leukapheresis was performed in one session in 163 (79.9%) patients; in 38 (18.8%) patients, two collection days were required to collect a sufficient number of CD34+ cells, and in 4 (2.0%) patients, three apheresis sessions were necessary. The mean number of leucocytes per kg body weight per reinfusion was 3.85x108 (SD 1.51x108; range: 1.16−8.67x108; median: 3.65x108). The mean number of CD34+ cells per kg body weight per reinfusion was 4.42x106 (SD 2.28x106; range: 0.99−15.00x106; median: 3.77x106).
Response rates and follow-up data
Response rates at the time of ASCT and on day 100 post-ASCT are presented in Table 1. Overall, 140 (68.6%) patients relapsed or had disease progression after the first ASCT. For 53 (30.9%) patients, no relapse or progression was reported after the first ASCT during the follow-up. The mean follow-up after the first ASCT was 74.9 months (SD 27.2; range: 0.0−141.3; median: 73.3). The mean PFS was 38.2 months (SD 27.3; range: 0.0−123.6; median: 33.3) and the mean OS was 63.7 months (SD 33.0; range: 0.0−221.1; median: 65.1) in our cohort. Among 82 patients (40.2%) who died, the most common cause of death was relapse of PCM (82.9%), followed by other reasons (8.5%) and circumstances related to HDCTx/ASCT (3.7%).
Response rates and follow-up data in patients with early versus late/no relapse
We performed a subset analysis of patients that were treated with ASCT more than 5 years before the data cutoff for these analyses (longer follow-up for the cohort). A total of 174 (85.3%) patients fulfilled these criteria and were eligible for further analysis. Of these, 55 (31.6%) died within 5 years after the first ASCT (group 1), resulting in an estimated 5-year OS of 68.4% (n=119) (group 2). The distinction between groups 1 and 2 was made in an attempt to identify clinical and graft features that were associated with worse outcomes (group 1: earlier relapse within 5 years post-ASCT) versus better outcomes (group 2: no disease activity within 5 years post ASCT). The proportion of male patients was 58.2% in group 1 and 67.2% in group 2. The mean age at the first ASCT was 58.2 years (SD 7.7; range: 37.6−68.9; median: 59.1) in group 1 and 56.6 years (SD 9.0; range: 28.8−70.8; median: 57.9) in group 2 (p=0.270). Induction therapy with PI was given to 60.0% of patients in group 1 and 62.2% of patients in group 2, while IMiD was given to 10.9% and 8.4% of patients, respectively. The combination of PI and IMiD was administered to 29.1% of patients in group 1 and 25.2% of patients in group 2. Thus, there was no association between the type of induction therapy and survival. The number of leukaphereses performed in groups 1 and 2 were one in 83.6% and 76.5% of patients, two in 12.7% and 21.8%, and three in 3.6% and 1.7% of patients, respectively. The mean number of leucocytes per kg body weight per reinfusion was 3.75x108 (SD 1.52x108; range: 1.16−8.52x108; median: 3.41x108) in group 1 and 4.01x108 (SD 1.59x108; range: 1.18−8.67x108; median: 3.83x108) in group 2 (p=0.312). There was no significant difference of mean number of CD34+ cells reinfused between group 1 (4.07x106 [SD 2.18x106]; range: 1.02−10.70x106]) and group 2 (4.56x106 [SD 2.24x106]; range: 0.99−15.00x106) (p=0.173). The median number of CD34+ cells per kg body weight per reinfusion was also not significantly different between groups 1 and 2 (3.44x106 vs 3.98x106; p=0.514).
Response rates according to IMGW criteria at the time of ASCT and on day 100 post-ASCT of groups 1 and 2 are presented in Table 2. At the time of ASCT, the overall response rate (ORR) was 94.5% (complete response [CR], very good partial response [VGPR] and partial response [PR]) in group 1 and was lower in group 2 (88%). In contrast, at day +100 post-ASCT, only 80% of patients in group 1 continued to display a response, whereas the ORR was 94.5% in group 2. Within the first 5 years post-ASCT in group 1, 48 (87.3%) patients experienced a relapse compared with 82 (68.9%) patients in group 2. Among patients who died within 5 years after the first ASCT (group 1), the most common cause of death was relapse (n=46, 83.6%), the remainder being due to ASCT-related adverse events in three (5.5%) patients and other reasons in four (7.3%) patients. In group 2, the major cause of death was relapse (n=97, 81.5%) and other causes (n=13, 11.1%).
Correlation of reinfused CD34+ cells with patient outcome
For this analysis, we only included patients who received a single ASCT (n=144). OS and PFS Kaplan-Meier curves of these patients are shown in Figures 1A and 1B. The mean number of reinfused CD34+ cells was 4.53x106 (SD 2.34x106; range: 0.99−15.00x106; median: 3.91x106); 25th percentile: 2.65x106; 60th percentile: 4.49x106; 75th percentile 5.80x106; 85th percentile 7.20x106; 90th percentile 8.04x106; 95th percentile 9.07x106. OS for the patients from 25th and 90th percentile was estimated using the Kaplan-Meier method (Figure 2).
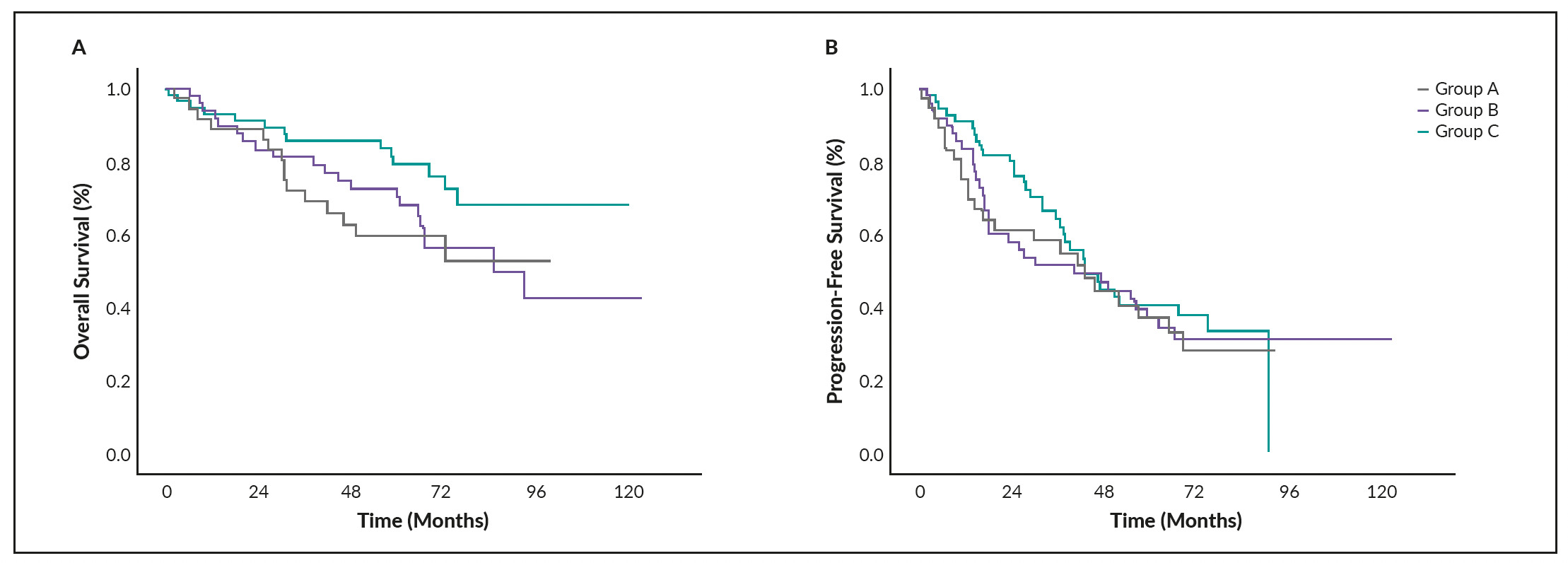
The OS curves of patients who received the mean number of reinfused CD34+ cells in selected percentiles showed comparable outcomes in those within percentiles higher than 75th. Based on this, patients were classified into 3 groups: Group A consisted of patients with ≤2.65x106 reinfused CD34+ cells (≤25th percentile; n=37), group B included patients with 2.66−4.49x106 (25th to 60th percentile; n=50) and group C included patients with >4.49x106 reinfused CD34+ cells (>60th percentile; n=57). OS and PFS for these three groups were estimated using the Kaplan-Meier method (Figures 3A and 3B). There was no significant difference between groups A−C regarding OS (p=0.103) and PFS (p=0.704).
Correlation of remission status at ASCT with patient outcome
The Kaplan-Meier estimator was applied to assess the correlation between the remission status at ASCT and survival outcomes (Figures 4A and 4B). Log-rank test showed no significant difference in survival curves correlating OS (p=0.786) or PFS (p=0.425) with different remission statuses.
Discussion
Here we present a monocentric descriptive analysis of 204 patients with PCM, assessing response rates to induction therapy, remission status post-HDCTx plus ASCT and characteristics of the apheresis product and their correlation with patient survival. Remission status improved from the day of ASCT to day 100 post-ASCT, as the percentage of patients with CR increased from 4.9% to 17.6% and the percentage of patients with VGPR increased from 40.2% to 52.0%. The lower CR in our analysis compared with previous studies (ranging between 29% and 46%)1 might be explained by the retrospective nature of real-world data versus prospective trial patient populations and by the introduction and broader application of novel agents in the induction regimen such as daratumumab23 or second generation PIs (carfilzomib)22 since the data cutoff date of our study, which have been shown to elicit deeper remission and longer remissions. Additionally, bone marrow biopsy as a requirement for response classification as CR according to IMGW was not performed in all patients analyzed, relying instead on negative serum IF and the disappearance of any soft tissue plasmacytomas. As a result, only patients with documented bone marrow biopsy and ≤5% plasma cells were classified as CR, leading to potential underestimation of the overall CR rate in our cohort. The measurement of minimal residual disease (MRD) is another milestone in evaluating treatment response and predicting long-lasting remissions, but was not available at our center at the time the patient cohort was treated.1,24
In the current study, 140 (68.6%) patients relapsed/progressed and 82 (40.2%) died during the follow-up. The median OS was 5.4 years and the median PFS was 2.8 years. These results are consistent with other studies reporting OS of approximately 4−6 years and PFS of 2−4 years using similar therapeutic regimens.8,22,25,26 The 5-year OS of 68.4% compared well with similar previously published results.27
We also studied the association of OS (p=0.786) and PFS (p=0.425) with remission status on the day of ASCT. Although the results showed no statistical significance, a trend toward improved OS and PFS in patients with either CR or VGPR compared with patients with PR could be observed.
We further investigated several potential factors affecting survival in PCM patients. The comparison of subsets of patients who died within 5 years after their first ASCT and those who had an OS of at least 5 years after their first ASCT showed no significant difference in the investigated variables between the two groups, including the number of reinfused leucocytes and the number of CD34+ cells reinfused with autograft. The latter presented a particular interest to elude a potential effect of contaminating residual myeloma cells in grafts on OS and PFS. The results showed that the estimated OS did not differ significantly in relation to the number of re-infused cells. Waszczuk-Gajda et al. (2018) investigated clonal plasma cell contamination in 59 patients using flow cytometry to examine its influence on survival and progression after auto-PBSC transplant and concluded that high clonal plasma cell contamination (>2.96x106) was associated with the worst PFS and OS outcomes.20 Similarly, in a prospective study by Gertz et al. (1997), tumor cells in apheresis products from 33 patients were quantified using immunofluorescence microscopy.28 Their results also showed that increased plasma cell counts (≥0.2x106) in the PBSC harvest were associated with a shortened PFS. It is conceivable that tumor cells contained in an autograft can seed new myeloma manifestations in a patient conditioned with HDC. We only used indirect measures of total leukocyte and CD34+ cell count, which did not compare our findings to these previous studies. One hypothesis could be that our products did not have clinically functional tumor cell contaminations in the apheresis product since we typically mobilize cells after 3 to 4 cycles of induction therapy, at a time when they already responded to treatment, and rarely mobilize without chemotherapy from a steady state. Alternatively, our data may indicate that in times of efficient second-line treatments and maintenance strategies, minimal tumor cell contamination no longer has a clinical impact on the survival of myeloma patients.
Our study has several limitations, including the retrospective character, which entails a dependency on the quality and completeness of the previously obtained clinical data of the patients. For example, documentation of posttransplant bone marrow biopsy was missing in many cases. In addition, the single-center character of this study further limits the study. Another important aspect that restricts the extrapolation of broader implications is the low patient number. Moreover, the risk group of the PMC patients according to Revised-International Staging System (R-ISS) was not considered in this study, leading to missing information that influences the estimation of the outcomes. Finally, patients who relapse following HDC and ASCT can have a variety of subsequent treatment regimens, comprising doublets or triplets of emerging new agents. The disadvantages of ASCT treatment are therefore obscured and may be no longer recognizable.
In conclusion, although our study did not show significant correlations, our findings are nevertheless relevant as they may point towards the decreasing impact of traditional disease parameters with outcomes in the age of novel agents. While ASCT remains the gold standard in the treatment of PCM, the landscape of innovative therapies is rapidly evolving, including potent agents for induction therapy that might perhaps obviate HDCTx or innovative cellular therapies, such as chimeric antigen receptor (CAR) T cells, genetically modified to target tumor cells. Currently, two CAR T-cell therapies directed against the B-cell maturation antigen (BCMA) are approved in Switzerland for the treatment of patients with relapsed or refractory multiple myeloma.29,30 These changes might eventually obviate HDCTx and ASCT altogether for PCM patients.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author Contributions
All authors contributed to and approved the final manuscript.
Acknowledgments
Editing assistance was provided by H+O communications Ltd., Zurich, Switzerland.
_(n_.png)
_for_selected_percentiles_of_reinfused_cd34____cells.png)

_overall_survival_(os)_and_b)_progression-free_survival_(pfs)_and_the.png)
_(n_.png)
_for_selected_percentiles_of_reinfused_cd34____cells.png)
_overall_survival_(os)_and_b)_progression-free_survival_(pfs)_and_the.png)