Established standard of care indications for CAR T-cell therapy in aggressive lymphomas
Commercial approvals
Tisagenlecleucel (tisa-cel; KYMRIAH®) was the first chimeric antigen receptor (CAR) T-cell therapy directed against CD19 that was approved in Switzerland for the treatment of adults with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy.1,2 The approval was preceded by U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) approvals, based on data from the pivotal, phase II JULIET trial, which demonstrated high rates of durable responses with tisa-cel in this patient population.3 Long-term data showed an overall response rate (ORR) of 53.0%, with 39% of patients having a complete response (CR) as their best overall response at a median follow-up of 40.3 months.4
Axicabtagene ciloleucel (axi-cel; YESCARTA®) was the first CD19-targeted CAR T-cell therapy that was approved by the FDA, with later approvals in Switzerland and the EU, for the treatment of R/R DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy,5,6 based on results from the pivotal ZUMA-1 trial.7 Long-term follow-up data from the ZUMA-1 study showed a median overall survival (OS) of 25.8 months and a 5-year OS rate of 42.6% among patients treated with axi-cel, with no new safety signals.8 In patients with an event-free survival (EFS) at 12 months, the 5-year OS rate was 90.9%. At the time of this analysis, 34% of all treated patients were still alive and received no subsequent therapy (excluding stem cell transplant) or retreatment with axi-cel, suggestive of durable long-term responses for these patients. Notably, the 5-year OS rate among complete responders was 64.4% and the median survival time was not reached.
Lisocabtagene maraleucel (liso-cel; BREYANZI®) is a more recently approved CAR T-cell therapy that is also directed against CD19 on B-cells.9 Like axi-cel,10 liso-cel is approved in Switzerland (again following FDA and EMA) for the treatment of R/R LBCL and PMBCL after two or more lines of systemic therapy.11 The approval was based on findings from the single-arm, open-label, multicenter phase I TRANSCEND NHL 001 study which demonstrated that R/R DLBCL patients treated with liso-cel (n=270) had a probability of continued response of 49.5%, with a median follow-up of 23.0 months.12,13 The estimated 2-year progression-free survival (PFS) and OS rates were 40.6% and 50.5% at a median follow-up of 23.9 months and 29.3 months, respectively. In terms of safety, no new signals occurred during the long-term follow-up and only a few adverse events (AEs) occurred after 90 days.
Real-world data on tisa-cel and axi-cel in patients with lymphoma
Tisa-cel and axi-cel have both demonstrated impressive clinical activity in the real-world setting. Efficacy outcomes of commercial tisa-cel for DLBCL appear comparable with the pivotal JULIET trial, including in patients not meeting study criteria. Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry showed an ORR of 57.4% in the overall population at a median follow-up of 17.2 months.14 As a best overall response (BOR), 42.4% of patients achieved a CR and 15% a partial response (PR). These response rates were similar among the JULIET-ineligible patients (ORR: 55.7%; CR: 41.6%), with both reflecting the rates reported in the JULIET trial (ORR: 53.0%; CR: 39.1%). Safety outcomes were more favorable in the real-world versus the trial setting.
In a large post-approval observational study of patients receiving axi-cel treatment (n=1,297), 57% of patients did not meet ZUMA-1 entry criteria, due to an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≥2 (5%), prior malignancy other than non-melanoma skin cancer (13%), cardiac comorbidities (13%), moderate to severe hepatic (2%), renal (2%), or pulmonary (28%) disease or other characteristics.15 At a median follow-up of 12.9 months, defined as the time from infusion to death or last contact, the ORR was 73% (CR: 56%) and the median duration of response (DoR) was not yet reached. The median PFS and OS were 8.6 months and 21.8 months, respectively. Both the ZUMA-1 eligible and ineligible patient cohorts had comparable outcomes. Response remained consistently positive across variables, except for ECOG PS of ≥2, which significantly impacted efficacy outcomes. Notably, advanced age (≥65 years) did not impact survival following axi-cel treatment (HR: 1.05 [95% CI: 0.88−1.26]), despite higher rates of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). These findings suggest that individual patient assessment for the standard of care axi-cel needs to account for comorbidities and individual risk-to-benefit considerations rather than strict adherence to ZUMA-1 study eligibility criteria or patient age.
Results were also reported from a large UK cohort of 300 patients with R/R LBCL who were successfully treated with CAR T-cell therapy (axi-cel: n=224; tisa-cel: n=76).16 Notably, patients receiving tisa-cel were significantly older and had a lower incidence of bulky disease at baseline and a higher lymphocyte count pre-lymphodepletion. At 3 months, the response rate was 48%, including a CR rate of 40% (42.0% for axi-cel, 34.2% for tisa-cel). Among evaluable patients (n=294), 37.8% had an ongoing CR at 6 months (39.9% for axi-cel, 31.6% for tisa-cel). The best ORR was 77% (CR rate: 52%) for axi-cel and 57% (CR rate: 44%) for tisa-cel. In terms of survival data, the 12-month PFS rate was 41.8% in the axi-cel cohort and 27.4% in the tisa-cel cohort. The median OS was 14.8 months in the treated population (axi-cel: 15.6 months; tisa-cel: 10.2 months), with a 12-month OS rate of 53.9% (57.1% vs 43.8%). Grade ≥3 CRS and ICANS occurred in 7.6% and 19.6% of patients receiving axi-cel and 7.9% and 3.9% of patients receiving tisa-cel, confirming the lower rates of ICANS in patients receiving tisa-cel treatment.
Furthermore, a multicenter retrospective study assessed usage patterns, safety and efficacy outcomes, and resource utilization of commercial axi-cel and tisa-cel in patients with R/R aggressive B-NHL treated in 8 US centers.17 Among the infused patients (n=240), those receiving axi-cel were significantly older than those receiving tisa-cel (p<0.001), while axi-cel recipients had a high comorbidity burden and were less heavily pretreated compared with tisa-cel recipients (p=0.020). The median time from apheresis to CAR T-cell infusion was significantly shorter for axi-cel versus tisa-cel recipients (28 days vs 45 days; p<0.001). Notably, 61% of patients treated with axi-cel and 43% of patients treated with tisa-cel would have been ineligible for the ZUMA-1 and JULIET trials, respectively. CAR T cells were infused in an outpatient setting in 8% of axi-cel recipients and 63% of tisa-cel recipients (p<0.001). Regarding safety, CRS and ICANS of any grade were significantly more frequent with axi-cel compared with tisa-cel (85% vs 39% and 56% vs 11%; both p<0.001), with rates of grade ≥3 CRS reported in 9% versus 1% of patients (p=0.017) and grade ≥3 ICANS reported in 38% versus 1% of patients (p<0.001). At a median follow-up of 12.4 months for the axi-cel cohort and 13.8 months for the tisa-cel cohort, comparable 12-month PFS rates (42% vs 32%; p=0.206) and 12-month OS rates (62% vs 59%; p=0.909) were observed. The day 90 ORR was 52% among patients receiving axi-cel and 41% among those receiving tisa-cel (p=0.113), including CR in 44% and 35% of patients, respectively (p=0.319).
In real-world clinical practice, no validated criteria exist that could help physicians choose among the different CAR T-cell products for a given patient, and a direct head-to-head comparison of the different products in a randomized clinical trial will likely be never performed. Nevertheless, real-world registry data may help answer some of the questions related to product-specific differences by using statistical methods that allow correcting for differences in patient populations undergoing a specific treatment. A comparison of axi-cel versus tisa-cel in R/R DLBCL patients was recently reported using real-life data from the French registry for commercial CAR T-cell therapy (DESCAR-T).18 Stringent 1:1 propensity scores (PS)-matching (n=418) demonstrated that patients receiving axi-cel versus tisa-cel had higher response rates (ORR: 80.4% vs 66.0%, CR rate: 60.3% vs 42.1%; p<0.001) at a 1-year median follow-up. Furthermore, axi-cel compared with tisa-cel significantly extended PFS (median, 8.2 months vs 3.1 months, HR: 0.61 [95% CI: 0.46–0.79]; p=0.0003) and OS (median, not reached vs 11.2 months; HR: 0.63 [95% CI: 0.45–0.88]; p=0.0072) (Figure 1). In terms of safety, axi-cel was associated with a higher incidence of ICANS (grade 1−2: 34.9% vs 19.1%; grade ≥3: 13.9% vs 2.9%; both p<0.001). These findings could help physicians with the individual risk-to-benefit analysis when choosing the CAR T-cell product for their patients. Independent validation of these findings in a different patient cohort is warranted.
Thus, a body of growing real-world data continues to support the conclusions from the registration trials and extends the safe and efficient use of CAR T-cell therapies also to patients who did not meet the strict clinical trial criteria. Additional registry studies will be needed and helpful in refining the clinical evidence necessary for optimal patient counseling and product choice in situations where more than one product is available in a specific indication.
Brexucabtagene autoleucel: First CAR T-cell therapy for mantle cell lymphoma
Brexucabtagene autoleucel (brexu-cel; TECARTUS®) is the first and so far the only CD19-directed CAR T-cell therapy that has been approved in Switzerland (following FDA and EMA approvals) for the treatment of adult patients with R/R mantle cell lymphoma (MCL) after at least two systemic lines of therapy, including a Bruton’s tyrosine kinase (BTK) inhibitor.19,20 This was due to positive results from the open-label, phase II, multicenter, single-arm ZUMA-2 trial, showing deep, rapid and durable responses, with 93% of patients having an objective response, including 67% with CR.21 Three-year follow-up data showed that these high response rates were sustained (ORR: 91%; CR: 68%), with a median DoR of 28.2 months and an ongoing response in 37% of patients (all CR).22 The median PFS was 25.8 months in all treated patients and 48.0 months in those with CR; the 24-month PFS rate was 52.9% and 71.8%, respectively (Figure 2). The median OS was 46.6 months in all treated patients and not reached in those with CR (30-month OS rate: 60.3% and 76.1%, respectively).
Real-world studies of brexu-cel are in line with the clinical trial data (ORR: 93%, 12-month PFS: 61%; 12-month OS: 83%)21 and further support its use in the treatment of R/R MCL.23–28 In a retrospective analysis of patients treated with brexu-cel across 12 U.S. academic medical centers (n=56), the ORR was 86% (n=56) and the estimated PFS and OS rates at 6 months were 77% and 88%, respectively.23 Notably, all eight patients with prior CNS involvement remained alive and free of relapse at the last follow-up. Comparable results were very recently reported from the U.S. Lymphoma CAR T Consortium (ORR: 89%, n=159), despite 78% of patients not meeting the ZUMA-2 entry criteria (>5 prior therapies, renal dysfunction, cytopenias, ECOG PS, CNS involvement and cardiac comorbidities).24 The median PFS was not reached and the median OS was 15.3 months, with 12-month PFS and OS rates of 54% and 75%, respectively. Similarly, a U.S. post-authorization safety study (PASS) demonstrated an ORR of 84% (n=106; 59% ineligible for ZUMA-2) in R/R MCL patients treated with brexu-cel.25 The median PFS was 8.9 months and the median OS was not reached; 6-month PFS and OS rates were 66% and 79%, respectively.
The current study landscape of CAR T-cell therapy in lymphoma: Moving to earlier lines and expanding indications
Axi-cel and liso-cel: Promising results in second-line LBCL
The current second-line standard of care (SoC) treatment in R/R LBCL after first-line chemoimmunotherapy (CIT) is salvage combination chemotherapy followed by consolidative high-dose chemotherapy (HDT) with autologous stem cell transplant (ASCT) for patients responsive to salvage chemotherapy.29 Results from the phase III, open-label, multicenter ZUMA-7 trial showed that, after prior treatment with CIT, CAR T-cell therapy with axi-cel was more effective than standard second-line treatment in patients with LCBL refractory to first-line CIT or who relapsed within the first 12 months of first-line treatment.30 The study met its primary endpoint of EFS, with interim results at a median follow-up of over two years demonstrating the median EFS of 8.3 months with axi-cel and 2.0 months with SoC (investigator-selected platinum-based CIT and HDT-ASCT) (HR: 0.398 [95% CI: 0.308–0.514]; p<0.0001) (Figure 3). Patients who were not eligible to receive HDT-ASCT after CIT salvage treatment went off-protocol and a large proportion of these patients went on to receive SoC CAR T-cell therapy in the third line. In the axi-cel arm, there was a 2.5-fold increase in the proportion of patients alive at two years that did not need additional cancer treatment or experience cancer progression (40.5% in the axi-cel group vs 16.3% in the SoC group). Among all randomized patients, the secondary endpoint of ORR was improved with axi-cel compared with SoC (83% vs 50%, odds ratio [OR]: 5.31 [95% CI: 3.1−8.9]; p<0.0001), including a respective CR rate of 65% and 32%. Axi-cel had a manageable safety profile consistent with prior studies. In a separate QoL analysis, significant and clinically meaningful improvements in QoL at Day 100 were observed for patients who received axi-cel (n=165) compared with those who received SoC (n=131).31 Faster recovery to pretreatment QoL for LBCL patients treated with axi-cel versus SoC was also reported. These data suggested that early treatment with CAR T-cell therapy may become a new standard in the second-line setting for LBCL patients that were refractory to first-line CIT or that relapsed within the first 12 months after first-line treatment. Indeed, FDA recently approved axi-cel for the second-line treatment of LBCL in this indication.32
In June 2022, the results of two preplanned subgroup analyses of ZUMA-7 were reported. An exploratory analysis investigating the influence of key prognostic markers on treatment outcomes showed that EFS was prolonged with axi-cel versus SoC in patients with high baseline tumor burden (HR: 0.289 [95% CI: 0.195–0.429]; p<0.0001) and elevated tissue hypoxia‑related lactate dehydrogenase (LDH) levels (HR: 0.324 [95% CI: 0.288–0.459]; p<0.0001).33,34 Whereas these tumor characteristics were associated with poorer EFS with SoC (HR high vs low tumor burden: 1.507; p=0.0240; HR elevated vs normal LDH: 1.556; p=0.0119), they did not impact responses with axi-cel (HR high vs low tumor burden: 0.915; p=0.6778; HR elevated vs normal LDH: 1.108; p=0.6132).
A separate analysis further showed that at a median follow-up of 24.3 months, axi-cel improved EFS also in patients aged ≥65 years (21.5 months vs 2.5 months with SoC; HR: 0.276 [95% CI: 0.164−0.465]; p<0.0001); the 2-year EFS rate was 47.8% and 15.1%, respectively.35,36 The ORR was 88% in the axi-cel arm and 52% in the SoC arm (OR: 8.81 [95% CI: 2.71−32.14]; descriptive p<0.0001) and the CR rate was more than doubled with axi-cel (75% vs 33%). The safety profile of axi-cel remained acceptable in this subset of patients.
Liso-cel was assessed as the second-line treatment of patients with primary refractory or early relapsed LBCL. In the phase III, randomized, open-label trial TRANSFORM, liso-cel was compared with SoC consisting of salvage CIT and HDT-ASCT in adults with R/R LBCL, who were refractory to or relapsed within 12 months of first-line CIT.37,38 Patients had not yet received treatment for relapse and were potential candidates for autologous HSCT. At a median follow-up of 6.2 months, the study met its primary endpoint of EFS (median, 10.1 months with liso-cel vs 2.3 months with SoC; HR: 0.35 [95% CI: 0.23−0.53]; p<0.0001). The CR rate, a key secondary endpoint, was almost doubled in the liso-cel arm versus the SoC arm at 66% versus 39%, respectively. PFS also favored liso-cel versus SoC; the median PFS was 14.8 months versus 5.7 months, respectively (HR: 0.41 [95% CI: 0.25−0.66]). Safety results in the second-line setting were consistent with previously reported data of liso-cel in the third- or later-line LBCL, with few severe CRS cases and neurological events (NEs). A QoL analysis from TRANSFORM also showed more favorable QoL results in patients treated with liso-cel compared with SoC.39 For example, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-30) cognitive functioning and fatigue group-level results exceeded the minimally important difference in the overall mixed model for repeated measurements (MMRM). Individual-level analyses showed that more patients improved and fewer patients deteriorated by month 6 in the liso-cel arm in most domains, including global health and fatigue.
Liso-cel also showed encouraging activity in patients with R/R LBCL not intended for HSCT. In the phase II PILOT trial on this patient population, liso-cel achieved an ORR of 80% and a CR rate of 54%, with a DoR of 12.09 months and 21.65 months, respectively.40 At a median follow-up of 13.0 months and 16.4 months, the median PFS and EFS were 9.03 months and 7.23 months, respectively. Despite patient frailty, no new safety signals were reported, with a low incidence of grade 3 CRS and NEs. In addition, patients experienced clinically meaningful improvements in EORTC QLQ-C30 fatigue and Functional Assessment of Cancer Therapy – Lymphoma Additional Concerns Subscale (FACT-LymS) scores.41 Based on TRANSFORM and PILOT data, liso-cel was recently approved by FDA for the treatment of adult DLBCL patients who are refractory or have relapsed within 12 months of first-line CIT or who have failed first-line CIT and are transplant-ineligible due to comorbidities or age.42
Tisa-cel was also investigated as second-line therapy in patients with aggressive B-cell lymphoma that was refractory or relapsed early after first-line therapy. The BELINDA trial was a phase III, randomized study of tisa-cel (n=162) versus SoC (n=160) in patients with R/R aggressive B-cell NHL within 12 months of first-line therapy.43 The trial did not meet the primary endpoint, showing that EFS in the tisa-cel arm is non-inferior to standard platinum-based chemotherapy. However, of interest, the tisa-cel arm had a higher percentage of patients with progressive disease at week 6 (prior to infusion) than the SoC arm (25.9% vs 13.8%).
Axicabtagene ciloleucel: A ray of hope in first-line LCBL
Notably, high-risk LCBL is a disease with a poor prognosis and a high unmet need for which approval of axi-cel has not yet been granted. Based on encouraging primary data from the phase II ZUMA-12 trial, the potential of axi-cel as a first-line treatment for patients with high-risk LBCL has now been realized.44,45 After a minimum of six months of follow-up, the ORR was 89%, with 78% of patients achieving a CR (n=29/37). Durable responses at a median follow-up of 15.9 months were also observed, with a median DoR, EFS and PFS not yet reached. The estimated OS rate at 12 months was 91%.
Novel combinations in third line LBCL
ZUMA-14: Axi-cel plus rituximab showed promise as dual antigen targeting therapy
In the third line and beyond, axi-cel also showed encouraging activity in combination with rituximab in the multicenter phase II ZUMA-14 trial (n=26).46 At a median follow‑up of 16.9 months, the overall response rate (ORR) was 88% and the CR rate was 73%, with 61% of patients in ongoing response (all CR). The median duration of response (DoR) was 17.6 months. The median progression-free survival (PFS) was 18.6 months, and the median OS was not reached at the data cutoff. The safety profile of axi‑cel plus rituximab was manageable, with no new safety signals.
Emerging landscape of CAR T-cell therapies in R/R follicular lymphoma
Patients with follicular lymphoma (FL) who are refractory to treatment or experience relapse have typically dismal prognosis and the approval of CAR T-cell therapy represents a potentially definitive treatment option. In Europe, R/R FL patients can receive tisa-cel2 after two or more lines of systemic therapy and axi-cel6 after three or more lines of treatment.
The phase II ELARA trial demonstrated that treatment with tisa-cel resulted in improved response rates in patients with R/R FL after two or more treatment lines or who relapsed after an ASCT.3,47 In the primary, prespecified interim analysis, the CR rate was 69.1% and ORR was 86.2% after a median follow-up of approximately 17 months. A recent subgroup analysis of patients with a high-risk disease from ELARA also showed promising efficacy responses.48 Tisa-cel was also associated with a 12-month PFS of 85.5% among patients who achieved a CR. Efficacy and durability of response were well maintained in all high-risk subgroups, except for the progression of disease within 24 months from the first CIT (POD24), high total metabolic tumor volume (TMTV) at baseline and the receipt of ≥5 prior lines of therapy. POD24 and high TMTV were independently associated with PFS. These results indicated that tisa-cel can induce high rates of durable response in high-risk patients having a poor prognosis with current non-CAR T-cell therapies.
The open-label, phase II ZUMA-5 trial demonstrated substantial and continued benefit of axi-cel in patients with R/R indolent NHL who previously had two or more lines of therapy.49 Among patients who were eligible for the primary analysis (n=104), the best ORR in the FL cohort (n=84) was 94% and 79% of patients achieved a complete remission at a median follow-up of 17.5 months. The study also met a key secondary endpoint, showing that among FL patients who received three or more lines of prior therapy and were eligible for the activity analysis (n=60), 95% had a response and 79% had CR. After a median follow-up of 30.9 months in patients treated with axi-cel as a third-line of therapy or beyond, 57% had an ongoing response and the estimated median PFS was 39.6 months.50
In a matching-adjusted indirect comparison (MAIC) analysis of ELARA and ZUMA-5 data after weighting, tisa-cel and axi-cel showed comparable efficacy (ORR: 92.59% vs 94.05%; p>0.05; PFS HR: 0.90; p=0.81).51 However, tisa-cel versus axi-cel was associated with a more favorable safety profile with regards to CRS (all grade: 45.52% vs 78.23%; grade ≥3: 0% vs 6.45%; both p<0.05) and neurotoxicity (all grade: 8.75% vs 56.45%; grade ≥3: 0.29% vs 15.32%; both p<0.05).
Outcomes from ZUMA-5 were further compared with SCHOLAR-5, an international, retrospective, external control cohort study, which applied key eligibility criteria from ZUMA-5.52 Propensity score methods were used to create a balance between ZUMA-5 (n=86) and SCHOLAR-5 (n=143), resulting in an effective sample of 85 patients in SCHOLAR-5. After a median follow-up of 23.3 months and 26.2 months, respectively, the median PFS was not reached in the ZUMA-5 cohort and was 12.7 months in the SCHOLAR-5 cohort (HR: 0.30 [95% CI: 0.18–0.49]; p<0.001), with 18-month PFS rates of 68.8% and 23.8%. The PFS benefit was consistent across subgroups including the patients who received ≥3 prior lines of therapy (HR: 0.20). The median OS was also significantly prolonged in ZUMA-5 versus SCHOLAR-5 (not reached vs 59.8 months; HR: 0.42 [95% CI: 0.21−0.83]). At 18 months, OS rates were 88.3% versus 67.1%, respectively, in the overall population and 88.3% versus 55.0%, respectively, among patients with ≥3 prior lines of therapy (HR: 0.31 [95% CI: 0.15−0.66]. The ORR was significantly higher in ZUMA-5 than SCHOLAR-5 (94% vs 50%; OR: 16.24; p<0.0001), with CR rates of 79% versus 30% (OR: 8.9; p<0.0001). Among patients who received ≥3 prior lines of therapy, OR was 28.1 for ORR and 15.4 for CR. A similar trend was observed in time to the next treatment (TNTT), with the median TNTT being not reached in the ZUMA-5 cohort and was 14.43 months in the SCHOLAR-5 cohort (HR: 0.42 [95% CI: 0.26–0.68]; p<0.001). These data show that axi-cel is superior to existing therapies for patients with R/R FL and offers a substantial clinical benefit in this clinical setting.
In addition to these studies, several ongoing trials are investigating CAR T-cell therapies in patients with aggressive lymphomas (Table 1). These include CAR T-cell therapies in various combinations with immune checkpoint inhibitors, such as nivolumab, atezolizumab and durvalumab, or other anti-cancer agents like BTK inhibitors.
New data on CAR T-cell therapies for B-cell acute lymphoblastic leukemia
Based on the results from the phase II ELIANA trial, tisa-cel was approved in Europe and Switzerland in 2018 for the treatment of pediatric and young adult patients ≤25 years of age with B-cell acute lymphoblastic leukemia (B-ALL) that is refractory, relapsed after transplantation or relapsed after two lines of therapy or later.1,2 In the final efficacy analysis at a follow-up of up to 5.9 years, the median relapse-free survival (RFS) was 43 months and the median OS was not reached, with 5-year RFS and OS rates of 44% and 55%, respectively.53 Results were comparable between pediatric and young adult patients. Among patients who achieved remission, 25% underwent allogeneic stem cell transplantation. In addition, the median time to B-cell recovery was 39 months and the probability of B-cell aplasia at 12 months was 71%. With this long-term assessment, there were no new treatment-related safety signals.
According to the CIBMTR study, real-world tisa-cel treatment in children and young adults with R/R B-ALL seems to be effective across all age groups and similar to findings reported from the ELIANA trial.54,55 At a median follow-up of 25.9 months, the ORR was 86.8% (vs 82.3% in ELIANA) and MRD-negative response was achieved by 97.9% of patients (vs 98.5%), with DoR of 61.4% (vs 67.4%). Also, the EFS rate at 12 months in the CIBMTR registry was comparable with that observed in ELIANA (52.6% vs 57.2%). The safety profile of tisa-cel in this study was generally more favorable than in ELIANA.
In this clinical setting, brexu-cel was also approved by the FDA for the treatment of adult patients with R/R B-ALL based on data of the phase I/II ZUMA-3 trial, which demonstrated a CR within 3 months from the infusion of 52% (28/54) that was estimated to exceed 12 months for more than half the patients.56 In the 2-year follow-up analysis of the phase II part, the median DoR among patients in complete remission (56%) was ≥20 months.57 In this population, the median OS was not reached, whereas it was 25.4 months for the whole cohort (n=55) and the pooled phase I and II cohorts (n=78). The OS benefit was observed irrespective of age or baseline bone marrow blast percentage but was less pronounced in patients with >75% blasts (14.2 months). The safety profile was manageable, with no new safety concerns or AEs of interest (CRS, neurotoxicity, or infections) since the primary analysis.58
Advances in CAR T-cell therapy to treat multiple myeloma
Idecabtagene vicleucel: The first CAR T-cell therapy approved in Switzerland for triple-class-exposed RRMM
In 2021, EMA and Swissmedic approved idecabtagene vicleucel (ide-cel; ABECMA®) as the first CAR T-cell therapy for the treatment of adult patients with relapsed/refractory multiple myeloma (RRMM) who have received at least three prior lines of therapies, including an immunomodulatory drug (IMiD), a proteasome inhibitor (PI) and an anti-CD38 antibody and have demonstrated disease progression on the last therapy.59,60 Approval of this B-cell maturation antigen (BCMA)-targeted therapy was based on positive results from the pivotal phase II KarMMa trial, a single-arm study in patients who had had at least three prior regimens and were refractory to their last treatment.61 At a median follow-up of 13.3 months, 73% of patients had a response and 33% had a CR or better (≥CR). The median PFS was 8.8 months.
A recent sub-analysis of the KarMMa trial aimed to identify pretreatment patient characteristics as predictors of CR by using a multivariate modeling analysis. Myeloma subtype (IgG heavy chain), high serum soluble BCMA as a biomarker for tumor load and elevated D-dimers and/or ferritin levels as markers of systemic inflammation were identified to negatively correlate with response to treatment and achieving a CR/stringent (s)CR. In the CAR T-cell product, a high vector copy number positively correlated with CR/sCR.62 These correlates are generally consistent with those previously reported with CD19-directed CAR T-cell therapies and may help physicians select patients more likely to achieve a deep and longer-lasting clinical response to ide-cel in real-world practice. Controlling tumor burden during the time needed for CAR T-cell manufacturing with an optimal bridging strategy may also benefit patients and help obtain CR/ sCR after ide-cel treatment.
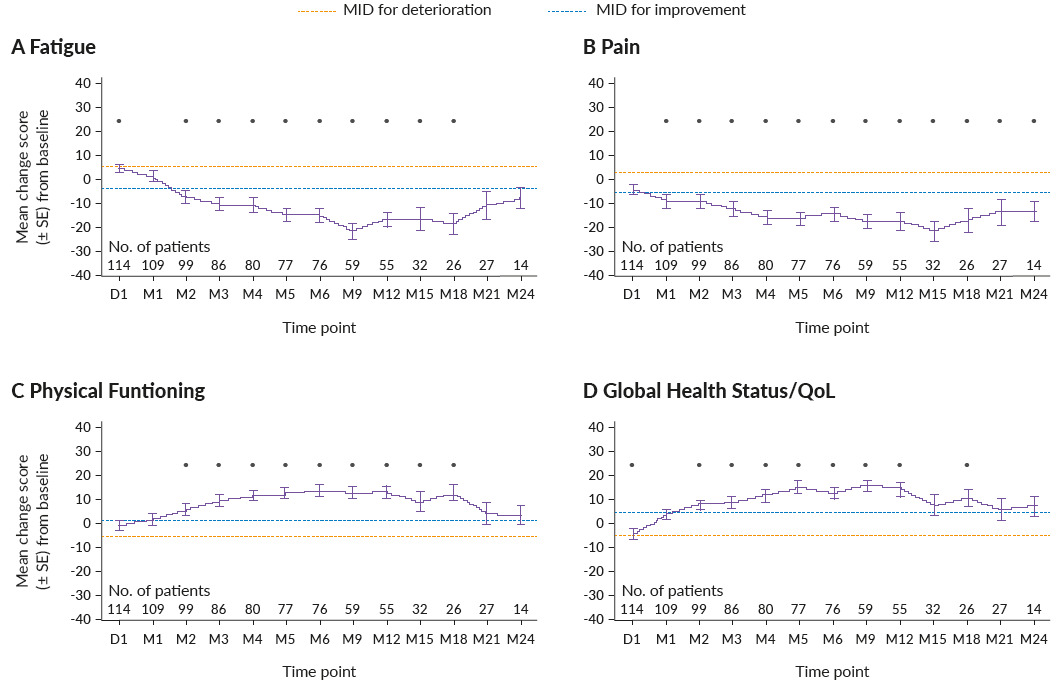
In addition to improvements in survival and clinical response outcomes, ide-cel has also previously shown clinically meaningful improvements in health-related quality of life (HRQoL) after nine months of follow-up in KarMMa.63 The impact of ide-cel treatment on HRQoL of triple-class-exposed RRMM patients in the KarMMa trial was assessed in an extended 24-month post-infusion study.64 From baseline, 40−70% of patients had clinically meaningful improvements in fatigue, pain, physical functioning and global health status/QoL scores, while 30−40% of patients experienced clinically meaningful improvements in cognitive functioning, disease symptoms and side effects (Figure 4).
In a real-world study of 196 heavily pre-treated RRMM patients, ide-cel showed comparable clinical outcomes to the KarMMa trial, although 77% of patients would not have met the eligibility criteria.65 Despite more patients having an extramedullary disease, a poorer ECOG PS, being penta-refractory and having received prior BCMA therapy, the best ORR was 86% (n=141) and the CR/sCR rate was 42% (KarMMa: ORR and CR: 73% and 33%) at a median follow-up of 13.3 months). Of note, 78% of complete responders were MRD negative. At a median follow-up of 5.3 months, PFS was 8.9 months and the 6-month OS rate was 84%. Safety data was generally consistent with those from KarMMa, with comparable incidence of CRS (any grade: ca 80% for both; grade ≥3: 3% vs 5%) and neurotoxicity (any grade: 8% for both; grade ≥3: 6% vs 3%), but higher tocilizumab (71% vs 52%) and steroid use (26% vs 15%) in the real-world setting.
CARTITUDE-1 and CARTITUDE-2 data continue to show promise for ciltacabtagene autoleucel in RRMM
In August 2022, ciltacabtagene autoleucel (cilta-cel; CARVYKTI®), a BCMA-directed CAR T-cell therapy with two BCMA binding sites, was approved in Switzerland for the treatment of adult RRMM patients after three or more prior lines of therapy, including a PI, IMiD and an anti-CD38 antibody, based on results from the pivotal, phase Ib/II, open-label CARTITUDE-1 trial.66–69 In the primary analysis at 12 months, ORR was 97%, including a CR rate of 67%. The 12-month PFS rate was 77% and the OS rate was 89%. Grade 3–4 hematological AEs were common and included neutropenia (95%), anemia (68%), leukopenia (61%) and thrombocytopenia (60%). CRS occurred in 95% of patients (grade 3 or 4: 4%). The spectrum of reported neurotoxicity was unique to cilta-cel and different from other CAR T-cell therapies to date.70 While ICANS occurred in 17% (all grade) and 2% (grade 3 or 4) of patients, a novel type of neurotoxicity was also documented. A total of 5% of patients experienced a cluster of cilta-cel-related movement and neurocognitive treatment-related adverse events (MNT) that required the development of a cilta-cel-specific algorithm for the prevention and early management of toxicities. Experiencing MNT-related neurotoxicity was more likely in patients with high tumor burden, with CRS grade 2 or higher, and any grade of ICANS after cilta-cel infusion. Measures to reduce the incidence of ICANS include the use of enhanced bridging strategies to lower tumor load before CAR T-cell infusion, as well as early intervention in the case of CRS and ICANS after CAR T-cell infusion. These and other implemented measures allowed to reduce the incidence of MNTs to <1% in cilta-cel trials.
A recent case study presented a patient treated in CARTITUDE-1 who developed neurocognitive and hypokinetic movement disorder with features of Parkinson’s disease approximately 3 months after cilta-cel infusion.71 Further analyses demonstrated BCMA expression and lymphocytic infiltration in the caudate nucleus, with the persistence of circulating CAR T cells in the blood and cerebrospinal fluid despite treatment with chemotherapy. The incidence of similar events with cilta-cel, as well as with ide-cel (grade 3 parkinsonism),72 support further investigation into the pathology of these treatment-emergent neurotoxicities to identify potential risk factors.
After a median follow-up of 18 months, cilta-cel continued to show a very high ORR of 97.9%, with an sCR achieved by 80.4% and a very good partial response or better (≥VGPR) by 94.8% of patients.73 The longer-term data showed no new safety signals, and there were no new events of cilta-cel-related MNT. A subgroup analysis of CARTITUDE-1 further showed that responses to cilta-cel were consistently high (ORR range: 95.1−100%) and durable across all prespecified subgroups, with a shorter median DoR observed in patients with International Staging System (ISS) stage III disease and baseline plasmacytomas.74 In the landmark 2 years post last-patient-in (LPI) analysis at a median follow-up: 27.7 months, cilta-cel demonstrated deep and durable responses (ORR: 97.9%; sCR: 82.5%; DoR: not estimable), with 91.8% of patients evaluable for minimal residual disease (MRD) achieving a negative status (threshold: 10-5).75 The median PFS and OS were not reached; the 27-month PFS and OS rates were 54.9% and 70.4% in the overall population and 78.8% and 90.8% in patients with sustained MRD negativity (≥12 months), respectively. The safety profile remained manageable, with no new treatment-related deaths.
Inverse probability of treatment weighting (IPTW)-adjusted comparisons of patient outcomes from CARTITUDE-1 (n=113) with diverse SoC therapies from real-world clinical practice (RWCP) (n=246) further demonstrated significantly improved clinical outcomes for triple-class-exposed patients receiving cilta-cel, including ORR (adjusted response-rate ratio [RR]: 4.43) and ≥VGPR (RR: 5.67), as well as PFS (HR: 0.15; p<0.001) and OS (HR: 0.38; p<0.001).76 In this study, more patients treated with cilta-cel experienced AEs, including grade 3−4 events, as compared with RWCP patients; however, the overall safety profile was manageable. In a similar study using the EMMY French cohort (n=309), cilta-cel was superior to RWCP after the inverse probability of weighting for average treatment effect among treated (IPW-ATT) adjustment (ORR RR: 5.1; ≥VGPR RR: 14.5; PFS HR: 0.15; OS HR: 0.21).77
Cilta-cel was also assessed in multiple myeloma (MM) patients in earlier lines of therapy in the phase II, multicohort CARTITUDE-2 trial, with a primary endpoint of MRD negativity following a single CAR T-cell infusion.78–82 MM patients who experienced early clinical relapse after front-line therapy that included a PI and an IMiD (cohort B, n=19) achieved an ORR of 100% and a ≥CR of 90% at a median follow-up of 13.4 months.79 Notably, 93.3% of MRD-evaluable patients (14/15) reached a negative status. The 12-month PFS rate was 89.5%. Similar results were observed in MM patients who had received at least 1−3 prior lines of treatment, including a PI and an IMiD, and were lenalidomide-refractory (cohort A, n=20).80–82 At a median follow-up of 17.1 months, the ORR was 95%, including a ≥CR of 90%, and all patients with MRD-evaluable samples (n=16) achieved negativity.82 The 15-month PFS rate was 70%. In both cohorts, cilta-cel led to early and durable responses (median time to first response: 1.0 month; DoR: not reached) that deepened over time.79,82
CONCLUSIONS
The application of CAR T-cell therapy spans a wide range of aggressive hematologic malignancies, such as diffuse large B cell lymphoma (DLBCL), relapsed and/or refractory (R/R) B-cell non-Hodgkin lymphoma (NHL), R/R multiple myeloma (MM), R/R follicular lymphoma (FL), B-cell acute lymphoblastic leukemia (B-ALL), all with limited therapeutic options. An extraordinary amount of promising clinical data, some of which have already resulted in new approvals, and growing real-world experience show that CAR T-cell therapies have already transformed the treatment landscape for many hematologic cancers. Such breakthroughs in the treatment landscape of aggressive hematologic malignancies are set to continue in the near future.
Conflicts of Interest
RS and AC declare no conflict of interest. CA has patents and pending patent applications in the field of engineered T-cell therapy, receives licensing fees and royalties from Immatics, and declares advisory/consulting roles and sponsored travel for Celgene/BMS, Gilead and Janssen.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author Contributions
All authors contributed to and approved the final manuscript.
Acknowledgements
Writing and editing assistance was provided by H+O communications Ltd., Zurich, Switzerland.
_in_patients_treated_with_commercial_axicabtagene_ciloleucel_(axi-cel.png)
_in_the_zuma-2_trial.png)
.jpg)
_in_patients_treated_with_commercial_axicabtagene_ciloleucel_(axi-cel.png)
_in_the_zuma-2_trial.png)
.jpg)