Introduction
Activating epidermal growth factor receptor (EGFR) mutations, which are found in exons 18−21 of the EGFR gene, are commonly observed in patients with non-small cell lung cancer (NSCLC), in particular in non-smokers and women.1 Their prevalence varies significantly among ethnic groups; EGFR mutation-positive tumors are found in 10–15% of Caucasians and 30–50% of Asian patients with NSCLC.2,3 EGFR-tyrosine kinase inhibitors (TKIs) are the gold standard for the treatment of sensitizing EGFR mutation-positive advanced NSCLC and have far greater efficacy compared with standard chemotherapy.4 In Switzerland, all three generations of EGFR-TKI are currently approved for the first-line treatment of patients with locally advanced or metastatic NSCLC with activating EGFR mutations.5 These include the first-generation reversible EGFR-TKIs, gefitinib6 and erlotinib7; the second-generation irreversible ErbB blockers, afatinib8 and dacomitinib9; and the third-generation irreversible EGFR-TKI, osimertinib.10 Recent studies have shown that second- and third-generation TKIs are more effective than first-generation TKIs.11–13 For example, osimertinib demonstrated survival benefits over first-generation TKIs and is associated with a more favorable safety profile, thus placing it as a standard first-line treatment option for patients with classical sensitizing EGFR mutations.14
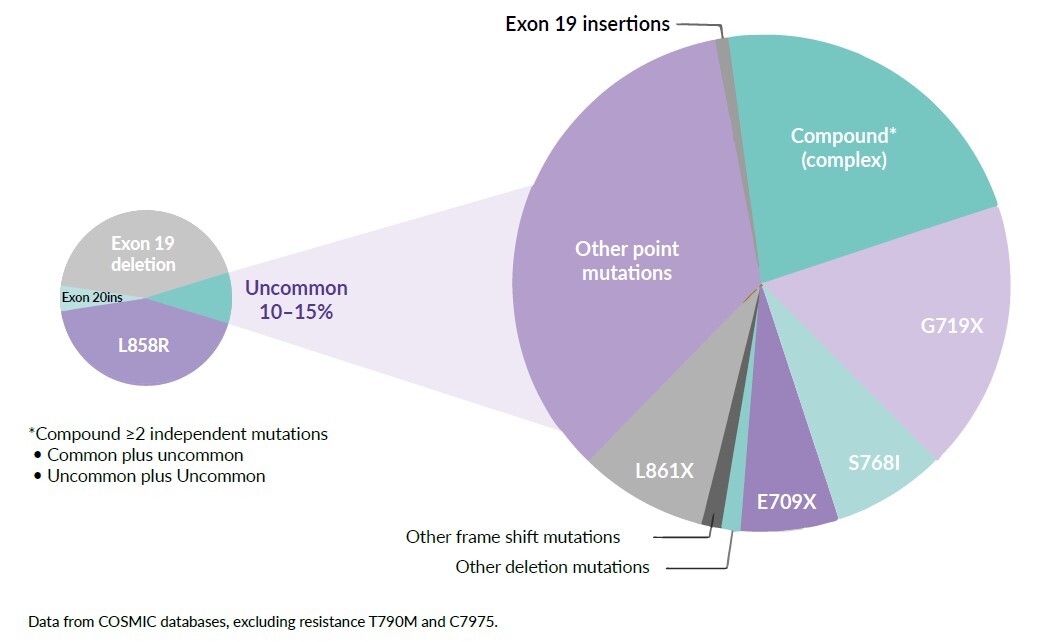
Recent improvements in detection methods have shown that uncommon or atypical EGFR mutations are more prevalent than previously anticipated, and a significant question remains regarding the treatment strategies for patients harboring the uncommon EGFR mutations. Furthermore, 25% of patients have complex or compound EGFR mutations that are defined as double or multiple independent mutations of the EGFR tyrosine kinase domain (TKD), in which an EGFR-TKI sensitizing or another mutation is identified alongside a mutation with unknown clinical significance (Figure 1).15,16 According to the Catalogue of Somatic Mutations in Cancers (COSMIC) database, >600 types of EGFR mutations have been reported.17 The heterogeneity of EGFR mutations in patients with EGFR mutation-positive NSCLC has potential clinical implications: different EGFR-targeted TKIs can have different activities against specific mutations.18,19 Little is known about the sensitivity of uncommon or compound mutations to a specific agent. Therefore, treating the uncommon EGFR mutations is a critical issue for daily clinical practice due to the granularity and heterogeneity of available data.
Uncommon EGFR activating or sensitizing mutation
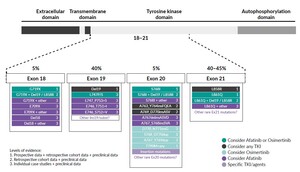
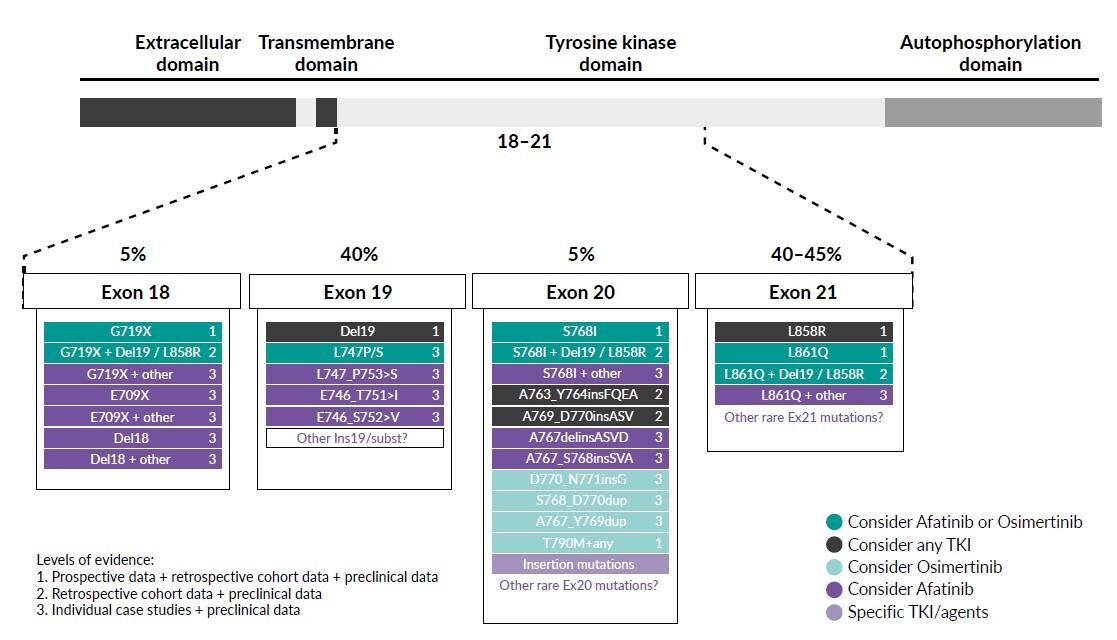
Exon 21 p.L858R point mutations and EGFR exon 19 deletions are collectively known as common sensitizing EGFR mutations, accounting for approximately 85–90% of EGFR gene mutations. Nearly 10–15% of the remaining patients have other mutation sites within or, more rarely, outside the kinase domain of the receptor and are classified as uncommon EGFR mutations (incidence ≤5% each) (Figure 1). Apart from exon 20 insertions, the major uncommon EGFR mutations are G719X (substitutions G719S, G719A, G719C and G719D), S768I and L861Q, which are found in exons 18, 20 and 21, respectively.20 These three mutations account for about 3%, 1% and 1% of all EGFR mutations in NSCLC patients, respectively.21 The positions of major uncommon EGFR mutations are depicted in Figure 2, showing the tertiary structure of EGFR gene. Rare mutations, especially G719X, frequently occur with other EGFR mutations. Exon 20 insertions are highly heterogenous and constitute the most common category of uncommon EGFR mutations and are primarily insensitive to EGFR-TKIs, especially second- and third-generation TKIs. Figure 3 describes the treatment recommendations for some major uncommon EGFR mutations and categories with documented clinical evidence and preclinical data.
Using the afatinib uncommon mutations database (n=693), a pooled analysis assessed the frequency of uncommon EGFR mutations among Asian and non-Asian patients with NSCLC.22 Among evaluable patients (n=298), 178 were Asians and 120 were non-Asians. The frequency of major uncommon mutations (G719X, L861Q and S768I) was 61.8% among Asian and 35.0% among non-Asian patients, while the frequency of exon 20 insertions was 16.3% and 39.2%, respectively. Furthermore, compound mutations were present in 14.6% of Asian and 6.7% of non-Asian patients, while the T790M mutation was detected in 10.7% and 11.7% of patients, respectively.
Efficacy of afatinib in the treatment of NSCLC patients with uncommon EGFR mutations
The activity of afatinib in TKI-naïve patients with advanced NSCLC who have tumors harboring uncommon EGFR mutations was investigated in a post-hoc analysis of prospectively collected data from patients who were given afatinib in a single-group phase II trial (LUX-Lung 2) and randomized phase III trials (LUX-Lung 3 and LUX-Lung 6).23 Of 600 patients who received afatinib across the three trials, 75 (12%) had uncommon EGFR mutations. Of these patients, 18, 16 and 8 had tumors harboring G719X, L861Q and S768I, respectively. The corresponding response rates were 77.8%, 56.3% and 100.0%, median progression-free survival (PFS) was 13.8 months, 8.2 months and 14.7 months, and the median overall survival (OS) was 26.9 months, 17.1 months and not estimable, respectively (Table 1). These results led to the approval of afatinib by the Food and Drug Administration24 (FDA) and European Medicine Agency25 (EMA) for patients with NSCLC with any sensitizing EGFR mutation (in addition to exon 19 deletions and L858R mutations). Afatinib is also authorized by Swissmedic8 as monotherapy for adult patients with advanced NSCLC, harboring EGFR activating mutations (exon 19 deletions, exon 18 G719X substitutions, exon 20 S768I substitutions, and exon 21 L858R substitutions and L861Q substitutions), who were not previously treated with EGFR-TKIs.
In a pooled analysis, Yang et al. (2020) further investigated the activity of afatinib in patients with tumors harboring uncommon EGFR mutations who were included in the afatinib uncommon mutations database (n=693) and treated in randomized clinical trials (n=75), compassionate-use and expanded-access programs phase IIIb trials and noninterventional trials (n=532) and case series or studies (n=86).20 Patients had uncommon EGFR mutations, which were classified into the following: T790M, exon 20 insertions, major uncommon mutations (G719X, L861Q and S768I, with or without any other mutation except T790M or an exon 20 insertion), compound mutations and others. In EGFR-TKI-naïve patients with available data (n=272/315), afatinib showed activity against major uncommon mutations (median time to treatment failure [TTF]: compound mutations [median TTF: 14.7 months; objective response rate [ORR]: 77.1%]; 10.8 months; ORR: 60.0%), other uncommon mutations (median TTF: 4.5 months; ORR: 65.2%) and some exon 20 insertions (median TTF: 4.2 months; ORR: 24.3%), with a median duration of response of 17.1 months, 16.6 months, 9.0 months and 11.9 months, respectively (Figure 4). Notably, among patients with tumors harboring compound mutations, the median TTF was prolonged in cases in which one of the mutations was a major uncommon mutation (median 16.6 months) (Figure 4). Of those, the median TTF was 14.7, 10.0 and 15.6 months in patients with G719X, L861Q and S768I mutations, respectively. The activity of afatinib was also seen in EGFR-TKI-pretreated patients (n=378).
However, this study had some limitations.20 In total, 86 patients included were published case studies, leading to potential publication bias because it is more likely that positive cases are published. Furthermore, EGFR mutation detection platforms vary greatly, and different testing methodologies were used in this analysis across studies; central testing was only done in the LUX-Lung trials. Furthermore, the database presently only contains information on patients who were treated with afatinib (vs other EGFR-TKIs). Nonetheless, the findings revealed that afatinib exhibited clinical activity against major uncommon and compound EGFR mutations in NSCLC patients. It also has broad activity against other uncommon EGFR mutations and some exon 20 insertion mutations.
Osimertinib for the treatment of NSCLC patients with uncommon EGFR mutations
Osimertinib is currently approved for the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC whose disease has progressed on or after EGFR-TKI therapy.10 This approval was based on the results from the open-label, phase III AURA3 trial, showing significantly greater efficacy of osimertinib compared with platinum-based therapy plus pemetrexed in patients with T790M-positive advanced NSCLC (including those with central nervous system metastases) in whom disease had progressed during first-line EGFR-TKI therapy.26 However, it has not been well-studied in NSCLC harboring less common EGFR activating mutations such as G719X, L861Q, S768I and exon 20 insertions, among others.27
Cho et al. (2020) reported the efficacy and safety of osimertinib in patients with NSCLC harboring uncommon EGFR mutations investigated in a multicenter, single-arm, open-label, phase II study in Korean patients (n=37).28 The mutations identified were G719X (n=19), L861Q (n=9), S768I (n=8) and others (n=4). Overall, the objective response rate was 50%, with a median duration of response (DoR) of 11.2 months. The median PFS was 8.2 months and the median OS was not reached. Of note, objective responses were seen in 77.8% of patients with the L861Q mutation, followed by 57.9% with G719X and 37.5% for S768I mutations. Thus, the results showed that osimertinib was associated with a high response rate, encouraging PFS and a long DoR with manageable toxicity in patients with NSCLC harboring uncommon EGFR mutations.
Comparable results were reported in a multi-institution, retrospective study assessing osimertinib in patients (n=51) with metastatic NSCLC who harbored at least one atypical EGFR mutation, excluding those with concurrent L858R, exon 19 deletions or T790M.29 Median time on osimertinib was 7.1 months in the overall cohort and 8.9 months in patients receiving first-line osimertinib. Patients harboring G719X (n=4) and L861Q (n=10) mutations had a median time on first-line osimertinib of 5.8 months and 19.3 months, respectively. In patients with exon 19 insertions (n=1), time on first-line osimertinib was 16.8 months. Thus, L861Q and exon 19 insertions appeared to benefit the most from osimertinib in this time-on-treatment retrospective analysis.
Osimertinib was also investigated in UNICORN, a multicenter, international, academic-initiated retrospective study that aimed to collect real-world data on the usage of osimertinib as the first-line EGFR inhibitor in patients with metastatic NSCLC harboring uncommon EGFR mutations.30 In fact, this is the largest dataset of osimertinib as the upfront EGFR-TKI in this patient setting. In a group of 46 patients, G719X was the most frequent mutation (35%), followed by de novo T790M (20%) and L861Q (15%). In addition, compound EGFR mutations were found in 35% of patients and baseline TP53 mutations in 28%. For 40 patients (87%), osimertinib was the first treatment given for advanced disease. Among evaluable patients (n=44), 5% had a complete response, 45% partial response and 39% of patients achieved stable disease, corresponding to a disease control rate of 88%. The median DoR was 17.4 months. The median PFS was 9.1 months and the median OS was 18.4 months. In terms of safety, the most frequent toxicities were gastrointestinal (52%) and skin (35%); 5 patients had grade 3−4 AEs. The UNICORN study continues to recruit patients, to expand the knowledge on the efficacy of osimertinib for this patient population.
Upcoming agents for the treatment of uncommon EGFR mutations in NSCLC
Neratinib is an oral, next-generation, pan-human epidermal growth factor receptor (HER) inhibitor that irreversibly inhibits the tyrosine kinase activity of EGFR, HER1, HER2 and HER4.31 Preclinical data indicated that EGFR exon 18 mutations are highly sensitive to neratinib, compared with the other approved first- and second-generation EGFR-TKIs.32 Recently, data were presented from the open-label, multicenter, phase II SUMMIT basket trial, which investigated the efficacy and safety of neratinib as monotherapy or in combination with other therapies in participants with HER (EGFR, HER2) mutation-positive solid tumors.33 This study also included an EGFR exon 18-mutant NSCLC cohort treated with neratinib monotherapy (n=11).33 Interim results showed an objective response rate of 40% among patients who were pretreated with a TKI, which was the primary endpoint of the analysis. Furthermore, the best ORR was 60%, the median DoR was 7.5 months and the median PFS was 9.1 months in this group of patients. In all patients, the median PFS was 6.9 months. This study showed a meaningful activity of neratinib in EGFR-TKI-pretreated patients with EGFR exon 18-mutant NSCLC, which is a group with very few effective options after disease progression. However, further evaluation of neratinib in this patient population is required in a larger dataset.
Amivantamab, an EGFR-MET bispecific antibody that confers immune cell-directing activity, is another potential therapy that is currently being explored in patients with NSCLC harboring uncommon EGFR mutations in Cohort C of the ongoing phase Ib CHRYSALIS-2 trial.34 In this study, amivantamab will be evaluated in combination with lazertinib, a highly selective, third-generation EGFR-TKI. The study also included NSCLC patients with exon 19 deletion or L858R mutations whose disease had progressed after treatment with osimertinib and platinum chemotherapy (Cohort A) and patients with EGFR exon 20 insertion-mutated NSCLC who progressed on platinum-based chemotherapy (Cohort B).
Treatment of NSCLC harboring EGFR exon 20 insertion mutations
EGFR exon 20 insertion mutations, typically located after the C-helix of the tyrosine kinase domain of EGFR, account for up to 5−7% of all EGFR mutations.35 EGFR is a 192-kbp gene consisting of 28 exons and located on the short arm of chromosome 7 (7p11.2).36 EGFR exon 20 insertions are grouped into two main categories: in-frame insertions or 3–21-bp duplications.37 In NSCLCs, approximately 90% of these insertions occur in the region encoding amino acids 766 to 775 (Figure 5), which form a loop between the αC-helix and β4 strand of the kinase domain, whereas other less frequent insertions occur within amino acids 761–766 that consist of the carboxy-terminal region of the αC-helix1.37
Generally, EGFR exon 20 insertions are associated with low or no sensitivity to the currently approved EGFR-TKIs.38 For patients with EGFR exon 20 insertion sequence variants (A763_Y764insFQEA and V769_D770insASV), treatment with erlotinib as first-line treatment is not recommended.35 Naidoo et al. (2015) suggested that these patients should be treated with chemotherapy in the first-line setting. In addition, patients with EGFR exon 20 insertions have similar clinical characteristics to those with common EGFR mutations but a poorer prognosis.39 Median survival of patients with exon 20 insertions was considerably shorter compared with patients with common EGFR mutations (16.5 months vs 33.0 months; p=0.06), but similar to the median survival (20.0 months; p=0.60) in EGFR wild-type cancers.
Efficacy of osimertinib in the treatment of patients with EGFR exon 20 insertion mutations
Osimertinib has shown limited antitumor activity in patients with EGFR exon 20-mutated NSCLC, with an ORR of 5% and PFS of 3.6 months.40 AEX20 (UMIN000031929), a phase I/II, single-arm, multicenter, open-label, non-randomized trial further showed an ORR of 0% and PFS of 113.5 days in advanced NSCLC patients with harboring exon 20 insertions who were treated with osimertinib. These results showed that a regular dose of osimertinib (80 mg) has limited clinical activity in NSCLC patients with EGFR exon 20 insertions.41
Preclinical data indicated that osimertinib is more likely to be effective against exon 20 mutants at a higher dose.42 The results from EA5162 (ECOG-ACRIN), a single-arm, phase II study of osimertinib (160 mg) in NSCLC patients with exon 20 insertions, showed that osimertinib was well tolerated and demonstrated clinical activity, with a response rate of 25%, disease control rate of 85% and median PFS of 9.6 months.43 However, it has been reported that osimertinib, either 80 mg or 160 mg once daily, showed less activity in Chinese NSCLC patients harboring diverse EGFR exon 20 insertion mutations. The median PFS did not differ significantly between patients who received osimertinib 80 mg or 160 mg once daily (2.5 months vs 1.3 months; p=0.161).44 In fact, patients harboring EGFR exon 20 proximal FQEA insertion (A763_Y764 and D770GY) variants showed numerically longer median PFS than those with other variants (4.2 months vs 2.2 months; p=0.164). A case report further showed a durable intracranial and extracranial response to osimertinib in a 44-year-old never-smoker woman with NSCLC and an EGFR exon 20 insertions (A763_Y764).45
Newly approved drugs and ongoing trials on EGFR exon 20 mutant NSCLC
In January 2022, Swissmedic approved amivantamab for the treatment of adult patients with metastatic or non-resectable NSCLC and EGFR exon 20 insertion mutations who have progressed during or after platinum-containing chemotherapy.46 The approval was based on CHRYSALIS, a phase I, multicenter, non-randomized, open-label, multicohort clinical trial.47 Patients received amivantamab once weekly for 4 weeks and every 2 weeks thereafter until disease progression or unacceptable toxicity. In the efficacy population (n=81), the ORR was 40% with a median response duration of 11.1 months. The median PFS was 8.3 months. In the safety population (n=114), the most common adverse events were rash (86%), infusion-related reactions (66%) and paronychia (45%).
In September 2021, FDA granted accelerated approval to mobocertinib, a first-in-class, potent, oral TKI, for adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.48 Approval was based on the 3-part, open-label, phase I/II non-randomized clinical trial with dose-escalation/dose-expansion cohorts.49 The primary analysis population was the platinum-pretreated patients (PPP) cohort. Efficacy was evaluated in 114 patients whose disease had progressed on or after platinum-based chemotherapy. Patients with a median 2 prior anticancer regimens received mobocertinib (160 mg orally, daily) until disease progression or intolerable toxicity. At a median follow-up of 14.2 months, the confirmed ORR was 28% by independent review committee (IRC) assessment and 35% by investigator assessment; the median duration of response by IRC assessment was 17.5 months. The median PFS by IRC assessment was 7.3 months. The most common treatment-related adverse events were diarrhea and rash. Mobocertinib is not yet approved by the EMA50 or Swissmedic.51
Currently, there are two ongoing first-line clinical trials for the treatment of patients with EGFR exon 20 mutations. The phase III PAPILLON trial aimed to assess the combination of amivantamab and carboplatin-pemetrexed therapy, compared with carboplatin-pemetrexed, in patients with advanced or metastatic NSCLC characterized by EGFR exon 20 insertions.52 Furthermore, the phase II EXCLAIM II trial is investigating the effectiveness of mobocertinib as first-line treatment in patients with locally advanced or metastatic NSCLC whose tumors harbor EGFR exon 20 insertion mutations.53
Chemotherapy and immunotherapy for the treatment of NSCLC patients with uncommon EGFR mutations
In clinical studies, chemotherapy was associated with significantly worse outcomes versus EGFR-TKIs in patients with common EGFR mutations54–58 but only sparse data are available for chemotherapy in NSCLC harboring uncommon EGFR mutations. A retrospective study showed that first-line chemotherapy was superior to first-line TKIs in terms of prolonged OS in patients with uncommon EGFR mutations, especially those with mutations in exon 18 and exon 20.59 Similarly, a real-world study in China demonstrated improved OS outcomes in previously untreated patients receiving chemotherapy versus EGFR-TKIs (median, 27.7 months vs 16.9 months).60
The efficacy of immunotherapy in NSCLC patients harboring uncommon EGFR mutations is not well defined, even though it has led to groundbreaking improvements in clinical outcomes of selected NSCLC patients.61–64 In general, immune checkpoint inhibitors (ICIs) are not recommended for the treatment of patients with EGFR mutations. A meta-analysis assessing the role of ICIs as second-line therapy in EGFR-mutated advanced NSCLC showed that ICIs significantly prolonged OS versus docetaxel in the EGFR wild-type subgroup but not in the EGFR-mutant subgroup.65 A retrospective study further indicated favorable PFS outcomes with immunotherapy in patients with uncommon EGFR mutations, including G719X in exon 18 and insertion in exon 20, and without T790M mutations compared with those with common EGFR mutations (median PFS, 256 days vs 50 days; p=0.003).66
Data also showed that ICIs in combination with chemotherapy tended to be more effective than ICIs alone in patients with pretreated NSCLC harboring EGFR mutations. In a retrospective real-world analysis, ICI plus chemotherapy was associated with improved ORR and survival outcomes versus ICI alone.67 In addition, the T790M mutation was a predictive biomarker for poor response to treatments comprising both ICIs alone and ICIs combined with chemotherapy. The efficacy of pembrolizumab plus chemotherapy was recently reported in a patient who was diagnosed with advanced lung adenocarcinoma with EGFR exon 20 insertion mutation and programmed death-ligand 1 (PD-L1)-positive tumors.68 The patient achieved disease control on this treatment regimen, suggesting that immunotherapy plus chemotherapy might be a potential treatment strategy for this clinical setting.
Conclusions
-
The first-generation EGFR-TKIs showed a variable and limited response in NSCLC patients with uncommon EGFR mutations. Interestingly, afatinib has demonstrated significant activity in point mutations or duplication in NSCLC patients harboring EGFR exon 18–21 mutations.
-
The management of EGFR compound mutations requires a tailored approach. For a compound of common plus uncommon EGFR mutations, afatinib or osimertinib might be suitable. In addition, afatinib could be a potential treatment approach for a compound of uncommon plus uncommon mutation.
-
EGFR exon 20 insertion mutations are a heterogeneous group of mutations and no specific exon 20 mutation drug has been identified to date. Currently, amivantamab and mobocertinib are the only two approved drugs for this patient population.
-
Emerging studies highlight new potential agents targeting EGFR uncommon mutations such as neratinib and amivantamab in combination with lazertinib.
Conflicts of Interests
Consulting or Advisory Role: BMS, AstraZeneca, Boehringer-Ingelheim, Roche, MSD, Pfizer, Eli Lilly, Astellas.
Speaker Bureau: Eli Lilly, AstraZeneca.
Author Contributions
The author crafted and approved the final manuscript.



_in_patients_with_tyrosine_kinase_inhibitor_(tki)-nave.jpeg)



_in_patients_with_tyrosine_kinase_inhibitor_(tki)-nave.jpeg)
