BACKGROUND
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, representing about 85% of all cases.2 Based on the pivotal SHARP trial,3 sorafenib, a multikinase inhibitor, was considered the standard of care for more than a decade. Recently, the pivotal IMbrave150 trial has shown a significantly prolonged progression-free survival (PFS) and overall survival (OS) with the combination of atezolizumab and bevacizumab compared with sorafenib in patients with unresectable HCC who have not received prior systemic therapy.4 As a result, this combination has become a standard of care as first-line therapy for patients without contraindications to either immune checkpoint inhibitors (ICIs) or antiangiogenic therapy. In addition, there are multiple first- and second-line systemic treatment options currently available for patients with locally advanced or unresectable HCC.
Due to positive results from the SHARP trial, sorafenib was the first approved oral tyrosine kinase inhibitor (TKI) as first-line therapy for the treatment of advanced HCC in 2007.3 More than ten years later, in 2018, another multitargeted TKI, lenvatinib, was found to be non-inferior to sorafenib based on the findings of the REFLECT trial.5 Concerning second-line therapies, regorafenib, another TKI, was the first therapy to show a survival benefit in patients whose disease progressed after the treatment with sorafenib in the RESORCE trial,6 leading to its approval in 2017. Two years later, Food and Drug Administration (FDA) approved cabozantinib based on the CELESTIAL trial, which demonstrated improved median OS compared with placebo in patients previously treated with sorafenib.7 Ramucirumab, a fully human monoclonal antibody (IgG1) directed against VEGFR2, showed a survival benefit only in the subgroup of patients with a baseline serum alpha-fetoprotein (AFP) level ≥400 ng/mL in the REACH-2 trial and is a therapeutic option in this setting.8 Immunotherapy is another option for HCC in second-line treatment. Based on the findings of the CheckMate 040 trial,9 nivolumab was the first approved immunotherapy for HCC, with an overall response rate (ORR) of 20% and a median survival of 16 months in patients who have progressed or were intolerant to sorafenib.
The advent of immunotherapy-based treatment regimens in the management of advanced HCC has the potential to improve patient outcomes, as demonstrated by IMbrave150 data discussed above. However, it also brings along new challenges, and the oncologist needs to apply careful patient selection and appropriately manage new toxicities in order to derive maximum clinical benefit from the pharmacological advancement.
CASE PRESENTATION
A 61-year-old man without any relevant medical history had a hepatological consultation for diffuse abdominal pain, dyspepsia and weight loss of 6 kg in 2 months. Gastroscopy and colonoscopy showed hyperemic edematous gastritis without erosions or active bleeding in the absence of esophageal varices. Histological analysis confirmed a normal gastric mucosa without histological alterations or the presence of Helicobacter pylori.
Laboratory analysis established a diagnosis of previously unknown chronic hepatitis C. Hemato-chemical examinations showed mild liver disease and mild anemia. The laboratory data was as follows: hemoglobin (Hb) 132 g/L (normal range, 140–180 g/L); serum aspartate aminotransferase (AST) 65 units/L (normal range, 10–50 units/L); gamma-glutamyl transpeptidase (GGT) 195 unit/L (normal range, 10–71 unit/L). Alpha-fetoprotein was 78.7 µg/L (normal range, <7.0 µg/L). Other laboratory values were within normal limits.
A hepatic magnetic resonance imaging (MRI) scan confirmed the presence of multiple HCC-compatible lesions, complete portal vein thrombosis (PVT) and partial thrombosis of the mesenteric vein (Figure 1).
Treatment with a prophylactic dose of apixaban, an oral inhibitor of factor Xa, was prescribed. The thoraco-abdominal computed tomography (CT) scan showed, in addition to the liver lesions, the presence of hepatic hilar, retroperitoneal and mediastinal lymphadenopathies, mild splenomegaly and minimal pelvic ascites (Figure 2). After a multidisciplinary discussion, the tumor was considered unresectable.
Taking into account the stage of the disease, the young age, the good performance status (PS) and OS benefit data in the IMbrave 150 trial, in the absence of absolute contraindications, we proposed a first-line therapy with atezolizumab plus bevacizumab. From the beginning of the treatment, the patient tolerated the therapy well and did not present treatment-related side effects. There was progressive improvement in liver parameters until the normalization of the values after the second cycle of therapy.
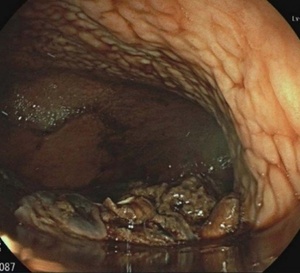
A few days before the fourth cycle of therapy, the patient was hospitalized for an episode of massive hematemesis resulting in a severe hemoglobin decrease with hemodynamic instability, secondary to rupture of esophageal varices. The patient was intubated and placed on mechanical ventilation. Gastric endoscopy was performed, which showed the presence of blood in the gastric cavity from suspected variceal bleeding (Figure 3).
The vasoactive agent, octreotide, was started concurrently with treatment with beriplex (human prothrombin complex), tranexamic acid and red cells transfusion. After prokinetic therapy, a second gastric endoscopy was performed, which showed the presence of esophageal varices F2−F3 (Figure 4).
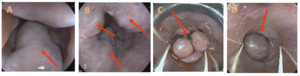
Endoscopic variceal band ligation (EVL) was performed with good results (Figure 5). In the absence of further bleeding, it was possible to extubate the patient after 4 days of hospitalization. The subsequent evolution was free of complications.
The patient started therapy with beta-blockers and underwent a follow-up endoscopy that showed the persistence of esophageal varices F2−F3, which were again subjected to band ligation (Figure 6).
Anticoagulation with dabigatran, a direct thrombin inhibitor, was initiated, a choice motivated by the availability of an antidote in case of future bleeding. After 17 days of hospitalization, the patient was discharged in good general condition.
We performed a new thorax-abdomen CT scan that showed excellent response to treatment, with an important reduction of the known liver lesions (Figures 7−8). There was also a significant reduction in the value of alpha-fetoprotein (from 131 μg/L at the beginning of the treatment to 7.2 µg/L after 3 cycles of therapy).
In view of the excellent response, we recently restarted the treatment with atezolizumab alone, as contemplated in the IMbrave150 trial. A restaging is expected shortly.
DISCUSSION
In the IMbrave150 trial, grade 5 adverse events (AEs) occurred in 15 patients (4.6%) in the atezolizumab-bevacizumab group and 9 patients (5.8%) in the sorafenib group.4 Grade 5 gastrointestinal hemorrhage was reported in 3 patients in the atezolizumab-bevacizumab group and none in the sorafenib group. Since bleeding is a known side effect of bevacizumab, an upper endoscopy for evaluation and treatment of varices was required 6 months before the enrolment.
The gold standard for the diagnosis of esophageal varices is esophagogastroduodenoscopy (EGD).10 In the present case, a gastric endoscopy had been performed about 2 months before the start of therapy and excluded the presence of esophageal varices. Nevertheless, the patient had rapid development of esophageal varices, probably secondary to portal hypertension due to portal vein thrombosis. Esophageal varices are caused by portal hypertension, most commonly due to cirrhosis or portal vein thrombosis,11 and the prevalence increases with the grade of liver cirrhosis. At diagnosis, around 40% of cirrhotic patients have esophageal varices.12 The incidence is around 5% at one year and 28% at three years.13 The annual risk of variceal bleeding for small varices (<5 mm) is 5% and increases to 15% in patients with large varices (>5 mm).14
Fortunately, systemic therapy followed by endoscopic variceal band ligation (EVL) provided hemorrhage control in our patient’s case. Without endoscopic intervention, the risk of rebleeding is almost 60%, with a 33% mortality rate.15 After acute variceal bleeding, the early mortality rate at 6 weeks is around 20%.16 Our patient overcame this serious complication through specialized medical management with blood transfusion, vasoactive agents, antibiotics and endoscopic variceal band ligation. Patients with hepatocellular carcinoma (HCC) generally present several comorbidities resulting in an increased risk for various complications. It is therefore essential to choose the most suitable treatments for these patients, taking into account their comorbidities and the risks associated with several therapies.
In IMbrave150, it was permitted to continue taking single-agent therapy in case of discontinuation of atezolizumab or bevacizumab due to an AE.4 In our patient’s case, we confirmed an excellent response after three cycles of therapy, and the AE experienced was entirely attributable to bevacizumab, which justified the decision to restart treatment with atezolizumab alone. A restaging is expected shortly.
CONFLICTS OF INTEREST
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors contributed to and approved the final manuscript.
Informed Consent
General written consent was obtained from the patient for the publication of this case report and any accompanying images.
Availability of data and materials
All patient data that support this case report are included in anonymized form in the published article.
_showed_complete_portal_vein_thrombosis_(pvt)_and.png)
_demonstrated_a_pathological_medi.png)
.png)
_(red_arrows).png)
_comparing_pre-_(panel_a__red_arrows)_and_.png)
_comparing_pre-_(panel_a__red_arrows)_and_.png)
_showed_complete_portal_vein_thrombosis_(pvt)_and.png)
_demonstrated_a_pathological_medi.png)

.png)
_(red_arrows).png)

_comparing_pre-_(panel_a__red_arrows)_and_.png)
_comparing_pre-_(panel_a__red_arrows)_and_.png)