Introduction
In the last two decades, the prognosis of breast cancer has steadily increased because of better diagnostic and therapeutic approaches. In early breast cancer, the introduction of third-generation chemotherapy and dose-dense regimes was the cornerstone for improved clinical outcomes.1 For early HER2-positive disease, treatment with chemotherapy plus trastuzumab and pertuzumab has resulted in improved prognosis,2 while in early triple-negative breast cancer (TNBC), the addition of immune checkpoint blockade to chemotherapy has led to superior pathologic complete remission.3 Recent data also demonstrated better outcomes in terms of event-free survival and overall survival (OS). In advanced HR-positive/HER2-negative breast cancer, a CDK4/6 inhibitor in combination with endocrine therapy is the current standard of care, as this combination treatment achieved prolonged OS compared with single-agent endocrine therapy.4 For advanced HER2-positive disease, there are many promising new agents, especially tyrosine kinase inhibitors and antibody-drug conjugates (ADC). In the DESTINY-Breast01 trial, trastuzumab deruxtecan, an ADC, achieved immense clinical benefit in pretreated patients with HER2-positive breast cancer.5 This drug is now extensively studied in several other indications, including early HER2-positive breast cancer and tumors with low HER2 expression. For advanced TNBC, state-of-the-art therapy in the first line is the combination of chemotherapy and immune checkpoint inhibitor in patients with PD-L1 positive tumor/immune infiltrate.6 Other options include poly (ADP-ribose) polymerase (PARP) inhibitors if pathologic BRCA mutations are detected.7 A new therapeutic alternative is sacituzumab govitecan, another ADC that showed superior efficacy and improvement in OS versus physician’s choice of chemotherapy in TNBC patients in the phase III ASCENT trial.8 This article summarized some important studies across all breast cancer subtypes and stages presented at ESMO Congress 2021.
Early HR-positive/HER2-negative breast cancer
In the early disease, therapy for HR-positive/HER2-negative breast cancer includes endocrine therapy with tamoxifen or an aromatase inhibitor. New clinical trial and meta-analysis data showed a great advantage of extended endocrine therapy (>5 years),9–13 but it is still not clear which patient subgroups benefit from extended endocrine therapy in the lymph-node negative setting. Recently, it has been shown that data using genetic signature as Breast Cancer Index (BCI) might help to determine which patient populations might benefit most.14
An Italian study presented at ESMO Congress 2021 aimed to examine the role of 5-year treatment with an aromatase inhibitor after 2−3 years of tamoxifen in postmenopausal patients with HR-positive breast cancer.15 A total of 2,056 patients with stage I−III breast cancer who previously received 2−3 years of tamoxifen were included. In the control arm, patients received 2−3 years of an aromatase inhibitor, while patients in the extended arm received 5 years of an aromatase inhibitor. The 12-year disease-free survival rate was 62% versus 67% in the control and extended arm, respectively (HR: 0.78 [95% CI: 0.65−0.93]; p=0.006). This effect was also observed in the multivariate Cox model including nodal status, tumor size, grading, age, hormone receptor status, HER2 status, previous chemotherapy and BMI (p=0.014). The 12-year OS was 84% in the control arm and 88% in the extended arm (HR: 0.77 [95% CI: 0.60−0.98]; p=0.036). The authors concluded that the results showed a clinical benefit of an extended aromatase inhibitor use after 2−3 years of treatment with tamoxifen and that this extended treatment should be carefully discussed with the patient. Tools like CTS5-based risk stratification might help to determine the risk of recurrence beyond 5 years.16 The role of other agents like CDK4/6 inhibitors in the high-risk population is still under investigation.
Advanced HR-positive/HER2-negative breast cancer
In advanced HR-positive/HER2-negative breast cancer, state-of-the-art treatment in the first and second line is endocrine therapy with aromatase inhibitors or selective estrogen receptor downregulators (SERDs) in combination with a CDK4/6 inhibitor.4 Currently, three CDK4/6 inhibitors are approved in this setting, including abemaciclib, palbociclib and ribociclib.17 At ESMO Congress 2021, the final OS analysis of the MONALEESA-2 trial on ribociclib was presented.18 In this study, 668 postmenopausal patients with metastatic HR-positive/HER2-negative breast cancer were randomized 1:1 to receive either ribociclib plus letrozole or placebo plus letrozole. After a median follow-up of 79.7 months, ribociclib plus letrozole showed a significant OS benefit versus placebo plus letrozole (median, 63.9 months vs 51.4 months; HR, 0.76 [95% CI: 0.63−0.93]; p=0.004), with estimated 6-year OS rates of 44.2% and 32.0%, respectively. No new safety signals were observed. For the first time, a CDK4/6 inhibitor showed significant OS benefit in the first-line setting of advanced HR-positive/HER2-negative breast cancer and based on these results, patients from this population should receive upfront ribociclib and letrozole.
Advanced HER2-positive breast cancer
In the past years, tremendous progress has been made in the treatment of advanced HER2-positive breast cancer, an aggressive subtype of the disease. Based on the CLEOPATRA study, the current standard of care in the first-line setting is chemotherapy with taxanes, trastuzumab and pertuzumab.19 In the second line, trastuzumab emtansine became the new standard following the results from the EMILIA study.20
During the plenary session of ESMO Congress 2021, data from the DESTINY-Breast03 trial were presented.21 In this phase III trial, trastuzumab deruxtecan was compared with trastuzumab emtansine in the second and later line in 524 patients. The primary endpoint of progression-free survival was met, with a median PFS not reached with trastuzumab deruxtecan and 6.9 months with trastuzumab emtansine (HR: 0.28; p=7.8x10-22). The confirmed overall response rate (ORR) was 79.7% in the trastuzumab deruxtecan arm versus 34.2% in the trastuzumab emtansine arm (p<0.0001). Rates of the most frequent side effects were comparable between the two treatment arms, while interstitial lung disease (ILD) was more common with trastuzumab deruxtecan. In conclusion, trastuzumab deruxtecan will have a major implication in HER2-positive disease and will change the practice for the second-line treatment. This drug is now extensively studied in several indications including the early HER2-positive breast cancers and tumors with low HER2 expression.
At ESMO Congress 2021, results from the phase III TULIP trial were presented, investigating [vic-]trastuzumab duocarmazine, another ADC, versus physician’s choice chemotherapy in 437 patients with pretreated locally advanced or metastatic HER2-positive breast cancer.22 The primary endpoint of the study was PFS. At data cutoff, the median PFS was 7.0 months with [vic-]trastuzumab duocarmazine and 4.9 months with chemotherapy (HR: 0.64 [95% CI: 0.49−0.84]; p=0.002). The most frequent adverse events with [vic-]trastuzumab duocarmazine were conjunctivitis (38.2%), keratitis (38.2%) and fatigue (33.3%). In conclusion, treatment with [vic-]trastuzumab duocarmazine significantly improved PFS in comparison with standard physician’s choice chemotherapy and may provide a new treatment option for patients with pre-treated locally advanced or metastatic HER2-positive metastatic breast cancer.
Advanced triple-negative breast cancer
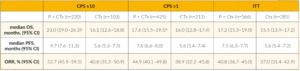
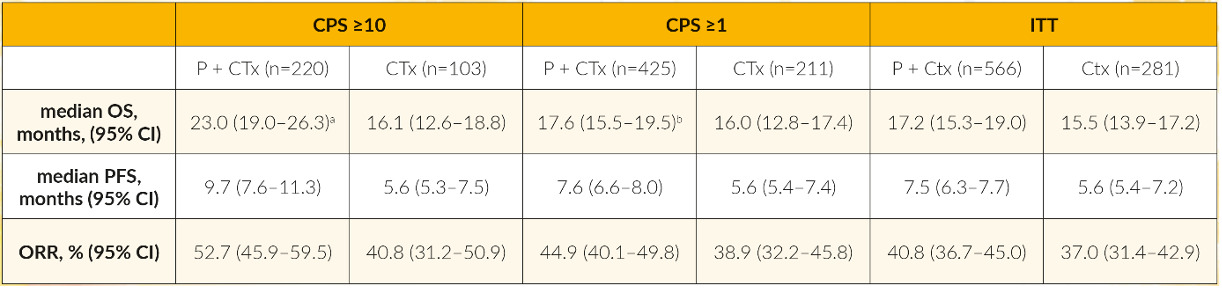
Although more than 90% of TNBCs harbor mutations in different pathways, less progress has been made in this clinical setting over the last 10 years and TNBC remains the breast cancer subtype with worse outcomes. Meanwhile, in advanced PD-L1-positive TNBC, therapy with immune checkpoint inhibitors became the standard of care. At ESMO Congress 2021, the final results with 44.1 months of follow-up from the KEYNOTE-355 study were presented.23 In this study, 847 patients with metastatic TNBC underwent 2:1 randomization to receive chemotherapy with or without pembrolizumab. Data indicated that chemotherapy plus pembrolizumab significantly improved OS versus chemotherapy alone in the subgroups of patients with combined positivity score (CPS) ≥10 (Table 1). The conclusion of the authors emphasizes the important role of an immune checkpoint inhibitor in PD-L1-positive tumors.