THE STRUCTURE AND DELIVERY OF THERAPEUTIC mRNA
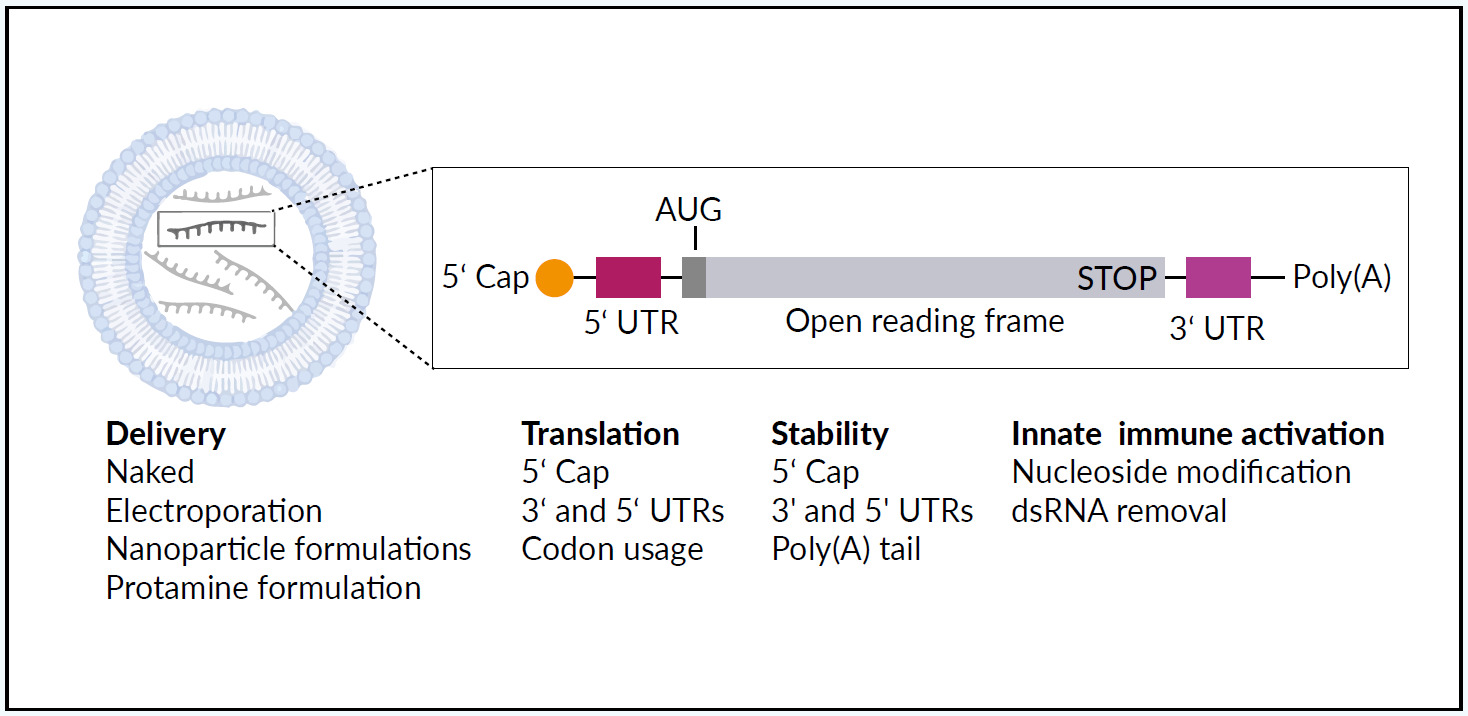
Synthetic mRNA acts as a template for translation of proteins, protein fragments, or peptides and has meanwhile found many pharmaceutical applications.1,15 Large-scale production of pharmaceutical-grade synthetic mRNA, mRNA optimization (for example the implementation of a very efficacious cap structure called CleanCap® by Trilink16), and the combination with nanoparticle-based formulations increase functionality.17,18 Transient and controlled production of encoded proteins from the mRNA template improves the safety and pharmacokinetic properties when compared with therapeutic recombinant DNA, viruses, or proteins. Figure 1 illustrates the structure and delivery of therapeutic mRNA using a lipid-based mRNA nanoparticle.1 The different mRNA delivery methods and structural elements can be “fine-tuned” to steer mRNA stability, translation, and the ability to activate an innate immune response. Recent developments in mRNA vaccines have been conducted in parallel with advances in cancer immunotherapy.
mRNA VACCINES AGAINST SARS-CoV-2 AND OTHER INFECTIOUS DISEASES
From December 2019, reports emerged from Wuhan, China, of an epidemic of novel coronavirus infection.3 Within weeks, this infection reached the USA and Europe.4,19 Coronavirus disease 2019 (COVID-19) is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel zoonotic RNA virus.3 Currently, infection with SARS-CoV-2 has involved all countries of the world, and the pandemic is still uncontrolled.5 In fact, in August 2021, New Zealand was put under strict lockdown again after the country’s first coronavirus case in 6 months was reported in the largest city of Auckland.
The pathogenesis of this new infection continues to be studied as new virus variants emerge and as long-term sequelae of infection, or “long-COVID”, become recognized. A population-based cohort study in Switzerland has reported concerning numbers as well as striking gender differences, with long-COVID rates rising up to 43.0% in women and 31.5% in men. Alongside the increasing transmissibility and mortality associated with novel variants of concern, these results underline the requirement for protective immunity. Early in 2020, vaccine development began, with the first emergency authorizations for novel mRNA vaccine from the end of 2020.6–12
Within a year after identifying SARS-CoV-2, Pfizer/BioNTech (Mainz, Germany) and Moderna (Cambridge, MA, USA) developed mRNA vaccines to SARS-CoV-2, BNT162b2 and mRNA-1273, respectively.6–9 Both consist of CleanCap® capped and nucleoside-modified RNA vaccine with a lipid nanoparticle formulation, which encodes a “pre-fusion” stabilized, membrane-bound SARS-CoV-2 full-length spike protein. BNT162b2 is administered at a dose of 30 μg followed by a second dose after 21 days.7 This mRNA vaccine received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) in December 2020,8 followed by the approval by Swissmedic in January 2021.20 The mRNA-1273 is administered at a dose of 100 μg followed by a second dose after 28 days. This vaccine received FDA EUA in December 2020,10 and Swissmedic approval in January 2021.21
Historically, vaccine development, the conduct of phase III randomized clinical trials, data analysis, and approval for these novel mRNA vaccines for COVID-19 were the most rapid for any new vaccine against a novel pathogen.6 This rapid response was made possible by using mRNA platforms that already existed for experimental vaccines against infectious disease (Table 1) and against cancer (Table 2), which have been available for the past two decades.1,11,12,22–24 and largely improved thanks notably to the optimization of liposomal formulations.17,18 As variants are spreading worldwide, studies are comparing the level of cross-neutralizing antibodies induced by classical BNT162b2 and ChAdOx1-S vaccinations with the heterologous prime and boost with ChAdOx1-S followed by BNT162b2, respectively. This study will analyze the safety and efficacy of the heterologous vaccination with ChAdOx1-S followed by BNT162b2.25 Recently, Pfizer and BioNTech have announced the submission of initial data to FDA to support booster dose of COVID-19 vaccine. The phase I safety and immunogenicity data show that the booster dose elicited significantly higher neutralizing antibody titers against the initial SARS-CoV-2 virus (wild-type), and the beta and delta variants compared to the levels observed after the two-dose primary series. Additionally, after the booster dose, neutralizing titers for variants were similar to wild-type. In fact, with the high levels of immune responses observed, a booster dose given within 6 to 12 months after the primary vaccination schedule may help maintain a high level of protection against COVID-19.26
mRNA-BASED VACCINES FOR CANCER IMMUNOTHERAPY
There are currently several vaccines that prevent infection with viruses associated with cancer, including those for human papillomavirus (HPV) and hepatitis B (HBV).28,29 However, the current rationale for developing cancer vaccines is to destroy established tumor cells rather than prevent cancer.1,28 So far, the promising results obtained with anti-cancer mRNA vaccines in preclinical developments were not supported by pivotal human clinical studies. However, during the past decade, development in multiomic analysis platforms, immune checkpoint inhibitors, and improved delivery systems, as well as an increased understanding of the tumor microenvironment, including antigen-presenting cells (APCs) and factors suppressing the immune response to malignant cells, have increased the potential of anti-cancer mRNA vaccines.27 Despite mRNA vaccines, synthetic mRNA coding for specific therapeutic molecules (receptors,30 cytokines,31 or antibodies32,33) can be used for anti-cancer therapies.34
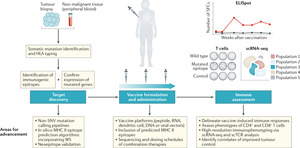
Figure 2 illustrates several mechanisms of anti-cancer immunomodulation by synthetic mRNA.1 Following vaccination, the isolated mRNA or vehicle-loaded mRNA vaccine leads to the expression of tumor antigens in APCs, which are presented to T cells in the context of the major histocompatibility complex (MHC) molecules.1,13,27,35 Both innate and adaptive immune responses can be generated as presented in the upper part. Passive immunity can be provided by synthetic mRNA (coding e.g., antibodies or immune receptors) as presented in the lower part. This review focuses only on the active immunity induced by mRNA vaccines.
The initial development of mRNA vaccines in oncology36 was limited by low mRNA immunogenicity.27 This limitation has been overcome by improving mRNA design (extremities, untranslated regions and coding sequence) and the carrier (mostly liposomes17,18), with the advantages of mRNA vaccines being their safety and nowadays efficacy, along with the ability to manufacture them on a mass scale.27,35 Currently, mRNA vaccines can be coupled with an immunologic adjuvant, which enhances the body’s immune response to antigens, and improved delivery strategies have been developed.13,37,38 Recent improvements in mRNA vaccines also include the modulation of mRNA-induced innate and adaptive immunity.13,35 The use of naked mRNA without a delivery vector may not provide a sufficient immune response as shown in the worldwide first clinical studies evaluating synthetic mRNA vaccines.22,39 Therefore, carrier-based mRNA vaccines have been developed, using lipid-based delivery, peptide-based delivery, polymer-based delivery, and cationic nano-emulsions, as well as dendritic cells.13
CURRENT STATUS OF mRNA VACCINES FOR CANCER IMMUNOTHERAPY
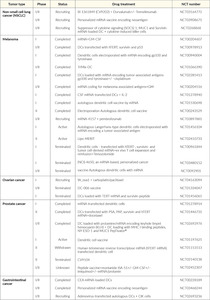
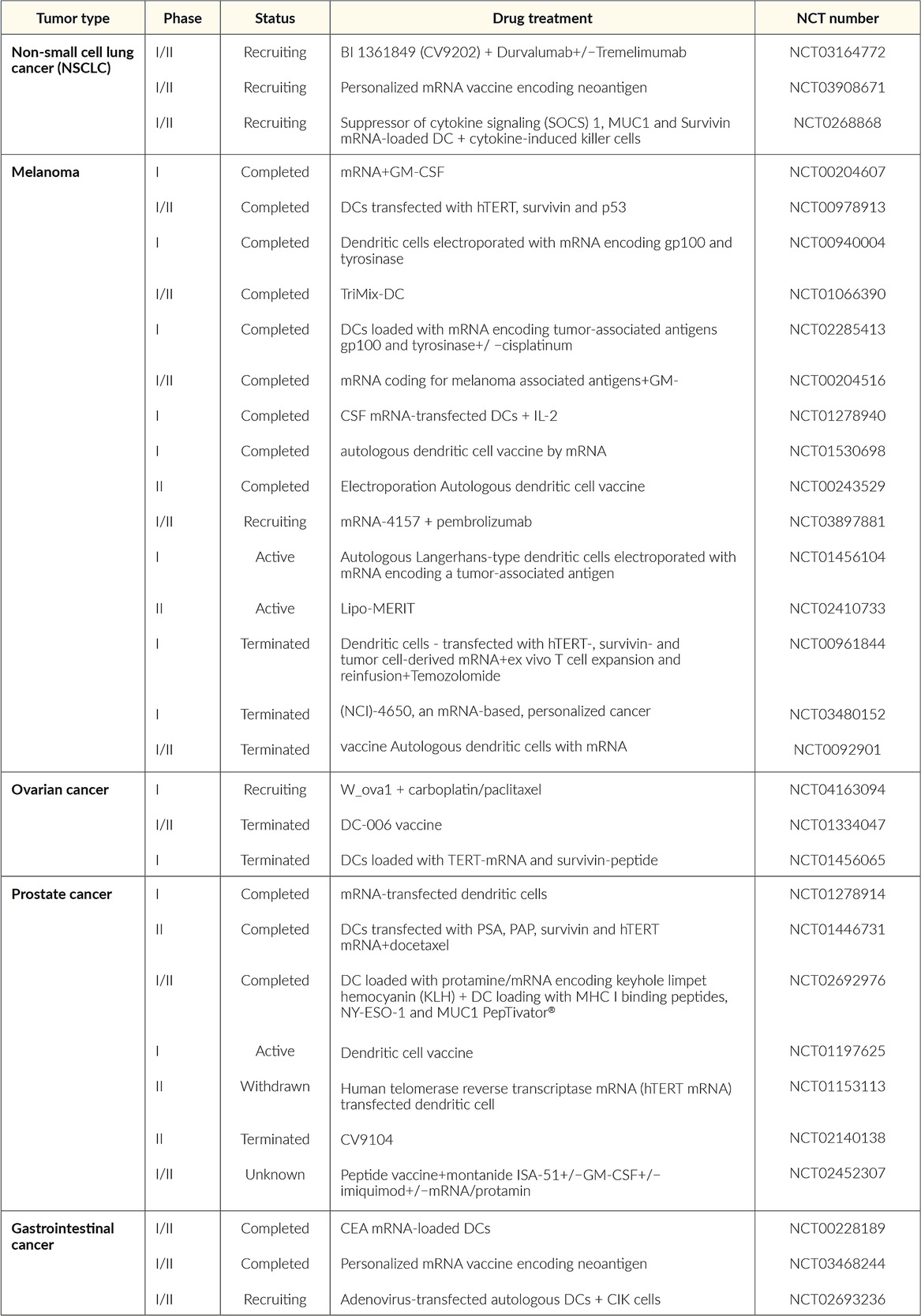
Future developments in mRNA vaccines in oncology are expected to include adaptations in the routes of administration and co-delivery of multiple mRNA vaccines with other therapeutic agents, such as checkpoint inhibitors as well as individualized vaccines (next-generation sequencing of cancer biopsies and healthy tissue allows to define mutations in the tumor for each patient and generate an adequate individualized vaccine).13,35 Table 2 summarizes the current status of mRNA vaccines in oncology that are undergoing or have completed randomized clinical trials. The following recent clinical trials of mRNA vaccines in cancer therapy have shown promising results in patients with solid tumors.
The Lipo-MERIT trial (non-modified mRNA, NCT02410733): BNT111
BNT111 is a liposomal mRNA (RNA-LPX) vaccine that targets four non-mutated, tumor-associated melanoma antigens (NY-ESO-1, MAGE-A3, tyrosinase, and TPTE), and is administered intravenously.40–42 Interim data were recently published from an ongoing, phase I, first-in-human, dose-escalation clinical trial that included 89 patients with advanced unresectable melanoma treated with repeated doses of BNT111 ranging from 7.2 µg to 400 µg.40 The most common adverse events (AEs) were mild-to-moderate, transient flu-like symptoms such as pyrexia and chills. The efficacy of BNT111 was assessed by imaging of metastatic lesions before and after vaccination in a subset of 42 checkpoint inhibitor-experienced patients with radiologically evaluable melanoma.42 At data cutoff, of 25 patients treated with BNT111 monotherapy, 3 achieved a partial response (PR), 7 had stable disease (SD), and 1 had complete metabolic remission of metastatic lesions. Of 17 patients treated with BNT111 plus anti-PD-1 therapy 6, experienced a PR. Treatment with BNT111 also resulted in the expansion and activation of circulating tumor-antigen-specific T cells with memory function exhibiting strong cytotoxic activity against tumor cells. Vaccine-induced antigen-specific memory T cells persisted for more than one year under continuous monthly vaccination.
RO7198457 (non-modified mRNA, NCT03289962): BNT122
RO7198457, an individualized mRNA vaccine that contains up to 20 patient-specific neoantigens, has been investigated as monotherapy and in combination with atezolizumab, a PD-L1 inhibitor, in patients with locally advanced or metastatic solid tumors, including breast cancer, prostate cancer, ovarian cancer, melanoma, non-small cell lung cancer (NSCLC), bladder cancer, and colorectal cancer (CRC).43,44 In a phase Ia dose-escalation study, 29 patients (median lines of prior therapies: 5) received RO7198457 at doses ranging from 25 µg to 100 µg.43 Of 26 evaluable patients, one patient with gastric cancer and metastatic liver lesions had a confirmed complete response (CR) that was ongoing for more than 10 months, while 12 patients achieved SD. Regarding safety, adverse events (AE) were mostly of grade 1−2 and included infusion-related reaction (IRR)/cytokine release syndrome (CRS), fatigue, nausea, and diarrhea. IRR/CRS were transient and reversible and presented primarily as grade 1−2 chills and fever. In the phase Ib portion of the study, 132 patients received RO7198457 at doses ranging from 15 µg–50 µg in combination with atezolizumab.44 The median number of prior therapies was 3 and 39% of patients received prior immunotherapy. Of 108 patients, one patient had a CR and 8 had PRs, while almost half of the patients achieved SD. Neoantigen-specific T-cell responses were observed in 77% of the patients. Most AEs were of grade 1−2 and included IRR, CRS, and influenza-like illness, fatigue, nausea and diarrhea.
RO7198457 is currently being investigated in combination with pembrolizumab, a PD-1 inhibitor, versus pembrolizumab alone in patients with previously untreated advanced melanoma in the phase II IMCODE001 trial.45
The KEYNOTE-603 trial (modified mRNA, NCT03313778): mRNA-4157
The KEYNOTE-603 trial evaluated the safety and efficacy of mRNA-4157 as monotherapy (n=16) or in combination with pembrolizumab (200 mg intravenously) (n=63) in patients with solid tumors.29 mRNA-4157 is a lipid encapsulated modified mRNA-based individualized cancer vaccine encoding neoantigens. Patients receive up to 9 cycles of mRNA-4157 by intramuscular injection at up to 1 mg alone or in combination with pembrolizumab. An updated analysis showed that in the head and neck squamous cell carcinoma (HNSCC) group (n=10) treated with the vaccine plus pembrolizumab, the overall response rate (ORR) was 50%, with 2 patients achieving CR and 3 achieving PR, all of which were ongoing. In addition, 4 patients had SD, resulting in a disease control rate of 90%. Median progression-free survival (PFS) was 9.8 months versus 2 months in pembrolizumab monotherapy. In the group of patients with microsatellite-stable (MSS) CRC (n=17), only 1 SD was reported and within 6 weeks of receiving the vaccine, 10 patients with MSS-CRC progressed, and 1 completed all doses. Only low-grade and reversible treatment-related AEs were observed.
The KEYNOTE-942 trial (modified mRNA, NCT03897881): mRNA-4157
In 2019, a phase I multicenter study reported on the safety, tolerability, and immunogenicity of mRNA-4157 alone in melanoma patients with resected tumors (n=13) and combination with pembrolizumab in patients with advanced or metastatic cancer (n=20).46 At a median follow-up of 8 months, the results showed that 11 patients on the monotherapy arm remained disease-free. In the combination arm, 1 out of 20 patients had a CR, 5 had PR and 6 patients had SD. All treatment-related AEs were reversible and of low grade, and no drug-related serious AEs or AEs grade ≥3 have been observed.
THE FUTURE OF mRNA VACCINES
The accelerated development, manufacture, clinical trials, and authorizations of mRNA vaccines have been propelled in the past year by the COVID-19 pandemic. An outcome from the current interest in mRNA vaccines highlighted their potential role in personalized medicine and therapeutics in oncology. All three major mRNA vaccine companies: BioNTech, CureVac and Moderna, have several ongoing clinical studies testing mRNA (modified or non-modified) vaccines against viral disease as well as vaccines against cancer. The latter uses a larger variety of technologies: the site of injection can be intradermal, intramuscular or intravenous, the mRNA can be coding for shared tumor antigens or be individualized to encode patient-specific mutations (Figure 3) and the vaccine can be used in combination with other anti-cancer treatments (mostly immune checkpoint inhibitors). It can be expected that some of those approaches will soon lead to the approval of anti-cancer mRNA vaccine modalities. The future will bring increasing collaboration between academic institutions, large pharmaceutical companies, and small biotech companies to develop and trial new mRNA vaccines. For example, Gritstone Oncology, a California-based biotechnology company, recently announced their findings from two phase I studies, which investigated two RNA products, viral prime plus self-amplifying RNA boost, GRANITE (GO-004), a personalized therapy with 20 tumor-specific neoantigens, and SLATE (GO-005), an off-the-shelf shared neoantigen therapy that includes 20 driver mutations in genes such as KRAS and TP53.47 In an early analysis, GRANITE showed good tolerability and robust neoantigen-specific CD8-positive T-cell responses consistently induced across all patients.47 GRANITE will be further evaluated in combination with immune checkpoint inhibitors nivolumab and ipilimumab in the phase II portion of the study, with an expanded cohort for patients with MSS-CRC and gastroesophageal cancer who have progressed on chemotherapy regimens. SLATE also demonstrated induction of CD8-positive T cells against multiple KRAS driver mutations, but strong responses were observed only in a subset of patients. The phase II portion of the GO-005 study will therefore assess the therapy in combination with nivolumab plus ipilimumab in patients with NSCLC harboring KRAS mutations who have progressed on prior checkpoint inhibitor therapy and patients with TP53-mutated advanced cancers.
The increasing optimization and clinical testing of mRNA vaccines mostly driven by the three major companies (CureVac created in 2000, BioNTech in 2008 and Moderna in 2010) led to the approval of very efficacious anti-COVID vaccines within a record time of one year from the disclosure of the sequence of SARS-CoV-2. Although the first studies of mRNA vaccines in human were performed in cancer patients,22,49,50 there is still no approved anti-cancer mRNA vaccine. However, results obtained lately in phase I studies are very promising and combination treatment (mostly immune checkpoint inhibitors combined with generic or individualized anti-cancer mRNA vaccines) are expected to be efficacious and soon approved. Meanwhile, accelerated by the COVID-19, mRNA vaccines against several pathogens are intensively developed to protect against infectious diseases not yet prevented by vaccines (e.g., infection by the respiratory syncytial virus [RSV], cytomegalovirus [CMV], human immunodeficiency virus [HIV], or malaria), or where the available vaccines may pose production/efficacy/safety issues [e.g., seasonal flu, rabies]). The mRNA vaccines being easy and quick to produce as well as vegan (production requires only bacterial-derived products and chemicals, no animal products), have now proven to be very safe and efficacious in the context of the COVID-19 pandemic. It has boosted the development of this vaccine format against many infectious agents and against cancer as well as to control autoimmunity (there, the vaccine specifically induces tolerance in the immune cells51). The versatility of mRNA vaccines offers a very broad spectrum of utilizations. Thus, it is expected that mRNA vaccines will, within the next year, offer new potent and safe medicaments against a growing number of diseases.
Conclusions
-
mRNA vaccines to prevent infectious disease and treat solid malignant tumors have undergone clinical development and clinical trials for several years.
-
During the COVID-19 pandemic, clinical development, clinical trials, and regulatory approvals of mRNA vaccines to SARS-CoV-2 have been accelerated.
-
mRNA vaccines in individualized oncology therapy rely on tumor tissue for gene sequencing to compare the patient’s normal transcriptome with their tumor.
-
A growing number of mRNA vaccines are expected to be validated against a broad spectrum of infectious diseases and cancers in the near future.
CONFLICT OF INTEREST
The author declares that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors contributed to and approved the final manuscript.