INTRODUCTION
The incidence of cutaneous squamous cell carcinoma (cSCC) is rising due to the increased longevity of at-risk patients.1,2 To date, approximately 20% of all non-melanoma skin cancers (NMSCs) that arise each year can be attributed to cSCC, and 2–5% of these will ultimately metastasize.3 Switzerland has one of the highest incidences of NMSC in Europe,4 with an estimated 25,000 patients per year affected.5 The rising incidence together with the non-negligible patient and economic burden and mortality rate highlights the importance and the clinical relevance of treatments for advanced cSCC.6
In Switzerland, there is currently no consensus first-line systemic treatment for patients who have locally advanced cSCC (lacSCC) or distant metastatic disease (mcSCC).7–9 There are several major classes of systemic agents used to treat patients, including immunotherapy, anti-epidermal growth factor receptor (EGFR) therapy, and chemotherapy.3 Recent studies with immune checkpoint inhibitors, also called programmed cell death protein 1 (PD-1) inhibitors, have yielded promising outcomes in advanced cSCC with approximately 50% objective response rates (ORR), and have led to the FDA, EMA and Swissmedic approval of the anti-PD-1 monoclonal antibody cemiplimab for unresectable high-risk cSCC.10–12 A systematic review of clinical studies with regard to efficacy, side effects and sustainability of therapeutic methods used in Swiss practice is therefore warranted.
The aim of these Swiss recommendations is to provide Swiss physicians with accepted, evidence-based decision support for the selection and implementation of systemic therapy in patients with locally advanced or distant metastatic cSCC. These recommendations may also improve standards-of-care in cSCC when patients are treated or managed in clinical practices outside Swiss centers of excellence. Study results of the recommended systemic therapies with regard to benefits and risks are also described to further support physicians in the decision-making process.
LEVEL OF EVIDENCE
The level of evidence for included studies is graded according to the Oxford classification (Oxford Centre for Evidence-Based Medicine 2011 levels of evidence).13
-
Level of evidence I: Meta-analysis, phase I and phase II cohort studies
-
Level of evidence II: Guideline adaptation, systematic review and meta-analysis, retrospective study
-
Level of evidence III: Review, prospective study, retrospective study, guidelines
-
Level of evidence IV: Case-series, case-controlled studies, or historically controlled studies
The grades of recommendations are classified as follows:
-
A: Strong recommendation (shall)
-
B: Recommendation (should)
-
C: Weak recommendation (may/can)
-
X: Should not be recommended
-
0: Recommendation pending: Not available currently or not enough evidence to give a recommendation in favor or against
DEFINITION OF CSCC
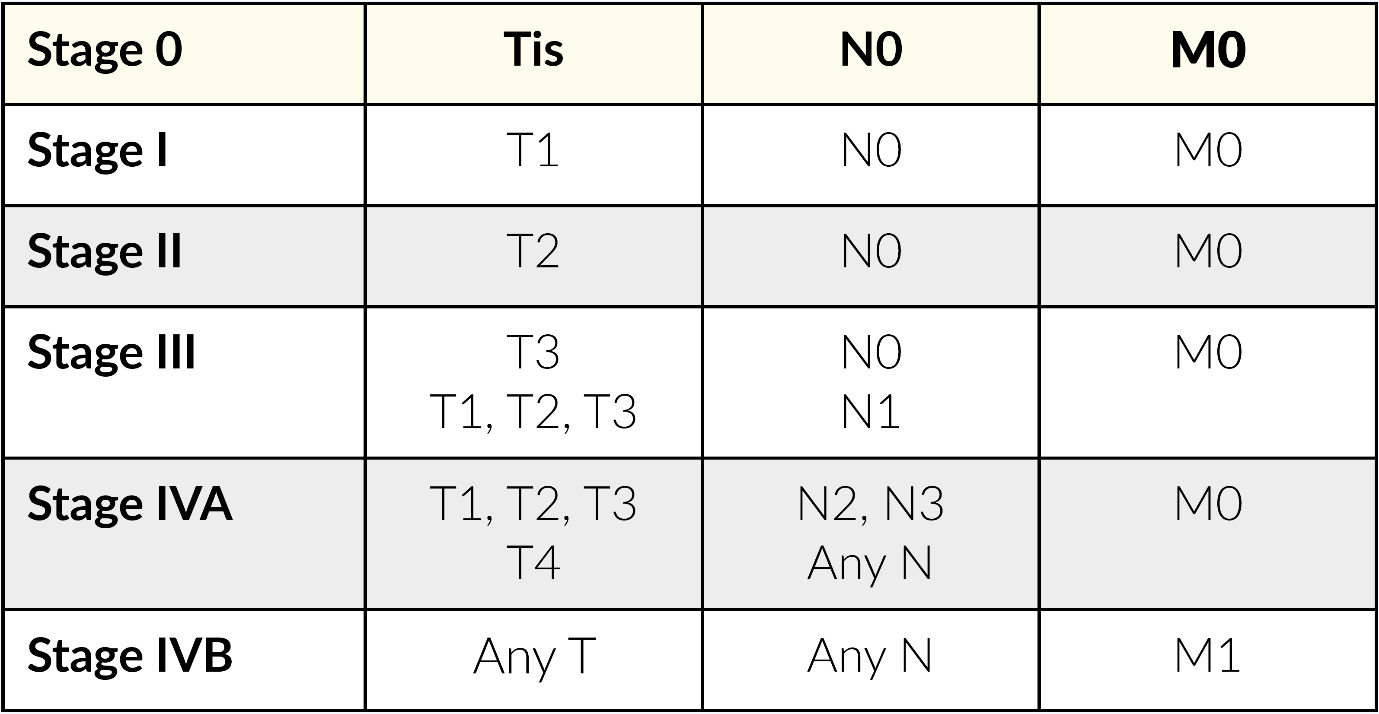
Herein, lacSCC shall be defined as non-metastatic cSCC, not amenable to either surgery or radiotherapy with reasonable hope for cure, because of multiple recurrences, large extension, bone erosion or invasion, or deep infiltration beyond subcutaneous tissue into muscle or along nerves, or else tumors in which curative resection would result in unacceptable complications, morbidity or deformity.14 Metastatic cSCC (mcSCC) includes loco-regional metastatic cSCC with in-transit metastases or metastasis to regional lymph nodes, or distant metastatic cSCC requiring systemic treatments.14 Table 1 shows the staging classification used for both lacSCC and mcSCC based on the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) tumor-node-metastasis (TNM) classification. This staging system is not without limitations, and Swiss physicians should only use this tool as part of an interdisciplinary approach to help classify patients into either low- or high-risk cSCC categories.
AN INTERDISCIPLINARY APPROACH IS NEEDED FOR TREATING CSCC PATIENTS
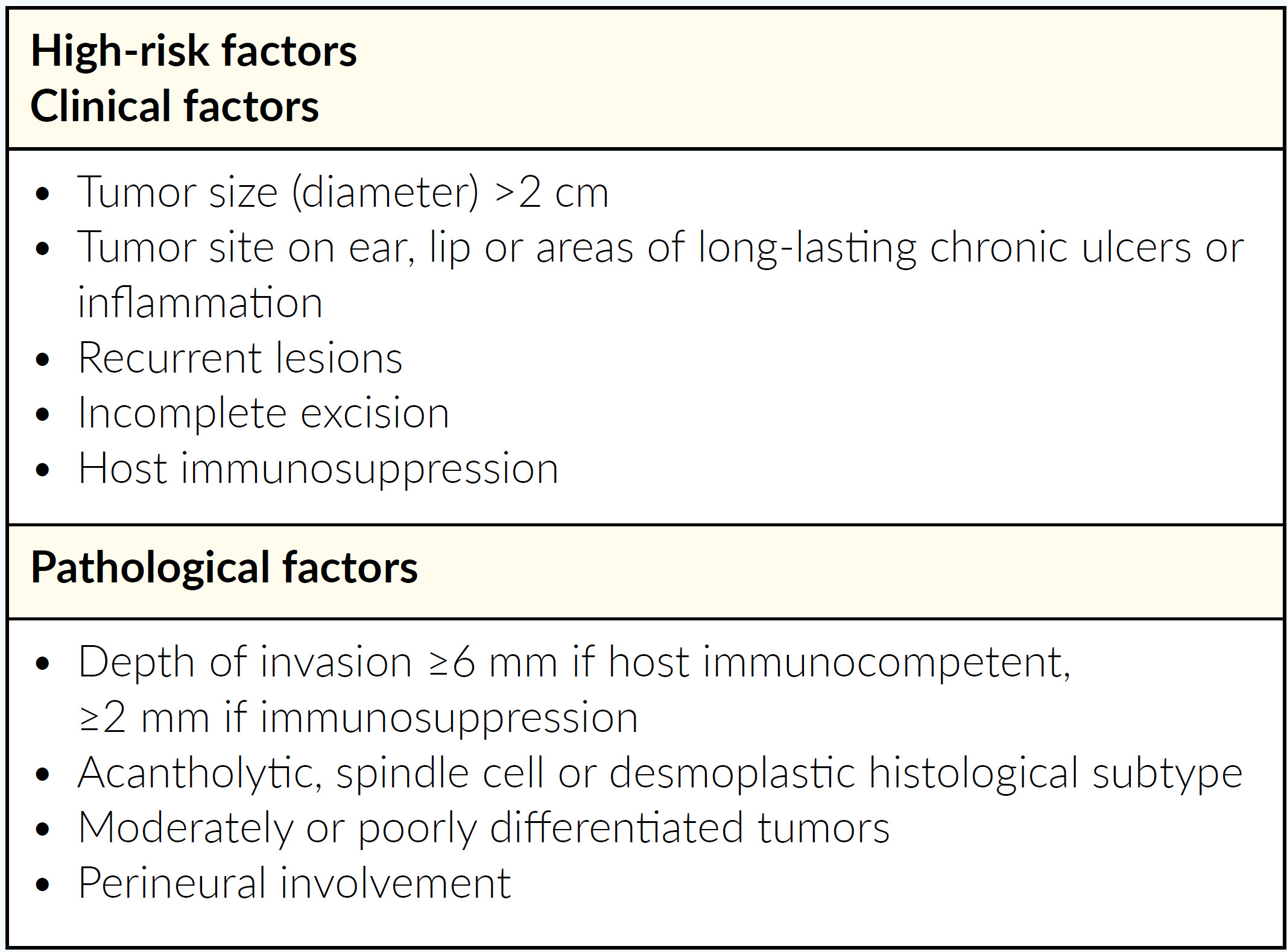
Every treating physician should start with classification of the cSCC to be treated, i.e. does the patient have a high or low risk tumor. Classification should also include whether the tumor is mcSCC or lacSCC. It is important to have experienced surgeons and radio-oncologists as part of an interdisciplinary tumor board to discuss and classify individual patient cases. All decisions for systemic therapy should be made by an interdisciplinary tumor board. Difficult-to-treat patients with lacSCC or mcSCC should be referred to and treated by specialized centers. Furthermore, and in addition to its definition and classification, it is important to note that tumor kinetics are often more rapid for lacSCC.7 For example, lacSCC often emerges from relapsing tumors, e.g. it can emerge as a fast, advancing tumor and/or as a rapid relapse (relapse within 3 months) of a previous successfully resected tumor. Various risk factors for high-risk tumors should also be considered by Swiss physicians, to help determine which at-risk patients should be considered for early systemic treatment (Table 2). The treatment management pathway for cSCC in Switzerland is shown in Figure 1.
TREATMENT OF COMMON PRIMARY CSCC
Briefly, the first-line approach for primary cSCC involves surgical excision of the tumor with careful assessment of skin margins regardless of the age group and anatomic location (Table 3).7 Postoperative assessment of the resection margins is required as standard-of-care during conventional surgery, with recommended 5mm or 6–10mm safety margins for low- or high-risk lesions, respectively.7 Conventional surgery is a preferred approach for low-risk cSCC tumors.7 En bloc excision with subsequent skin grafting is a suitable option for patients with a cluster of multiple cSCCs (e.g. on the scalp).7 Re-excision of positive margins should be performed for all operable cases (Table 3).7
There are two main types of surgical procedure, conventional surgery and micrographically controlled surgery (MCS), both are indicated regardless of patient age and anatomic location of the tumor.7
MCS is a particularly effective treatment for high-risk cSCC.7 It involves removing serial horizontal sections of the tumor margin in order to spare as much tissue margin as possible while minimizing the risk of recurrence.7 Two techniques of MCS are available in Switzerland: MMS (Mohs Micrographic Surgery) which uses frozen sections, and 3D histology or “slow Mohs” which uses paraffin sections.7 Surgical removal by MCS is more time-consuming, labor-intensive and therefore more expensive, but achieves higher rates (>90%) of R0 resection (microscopic disease-free margins) and lower rates (≤4% vs. 3.1–8.0%) of recurrence, compared to conventional surgery.7 For these reasons, MMS may be preferred to conventional surgery for excision of certain cSCCs, e.g. those on the head and neck with a high recurrence rate.7 Another major advantage of MMS is same-day tumor removal and reconstructive surgery.7
Regular physical examination, including inspection of the entire skin and inspection and palpation of the excision site, the in-transit route and the regional lymph nodes, should be part of the follow-up for all patients.7 For patients with a low risk of recurrence or new skin cancers, it is recommended that they have a clinical examination every 6–12 months for 5 years.7 For high-risk primary cSCC, e.g. for patients with a risk of local recurrence or new skin cancers and risk of regional metastases, follow-up every 3–6 months for 2 years or every 6–12 months for 3 to 5 years, respectively, is recommended, and annually thereafter.7
SURGICAL TREATMENT FOR REGIONAL NODAL DISEASE
Following clinically or radiologically detected lymph node-positive cSCC, i.e. confirmed with cytology or biopsy, radical lymph node dissection of all affected areas should be performed (Table 3).7 The interdisciplinary tumor board should decide the extent of dissection for each patient case, as well as nonoperative therapies when surgery is contraindicated or if the patient refuses surgical treatment.7 Due to the rarity of nodal metastases, elective lymph node dissection is not recommended for lymph node-negative cSCC.7 For patients with high risk of regional and distant metastases, follow-up every 3 months for 5 years and every 6–12 months thereafter is recommended.7
NON-SURGICAL TREATMENTS FOR SELECTED PRIMARY CSCC CASES
In Switzerland, alternative destructive modalities such as curettage and electrodessication, photodynamic therapy (PDT), cryotherapy and lasers are not recommended for primary invasive cSCC.7 For non-invasive, i.e. in situ, cSCC, these approaches could be used.
ADJUNCT RADIOTHERAPY
Radiotherapy is a considerable alternative for patients with primary cSCC for whom curative surgery is not possible (e.g. due to comorbidities, or if the patient declines surgery) or when surgery could cause disfigurement or a poor functional outcome (Table 3).7 For small cSCCs (e.g., diameter <1cm), definitive primary radiotherapy is a suitable alternative to surgery.7 Long-term effects are very good in some areas, e.g. the periocular region, but less so for areas such as the ear. For regional nodal metastases and extracapsular extension of head and neck cSCCs, adjuvant radiotherapy should be considered (Table 3).7 Postoperative radiotherapy is also an option for cSCC with positive margins after surgical excision if re-excision is not possible.7
SYSTEMIC TREATMENTS FOR ADVANCED CSCC
It is important to determine which cSCC patients should receive systemic therapy after interdisciplinary tumor board discussion. For example, patients with advanced cSCC, including regional node involvement or metastases to distant tissues or organs, may not respond to surgery or radiation, so earlier systemic therapy is needed.7 Systemic treatment options include immunotherapy, epidermal growth factor receptor (EGFR) inhibitors, chemotherapy (platinum-based chemotherapy was used as the standard of care in the past), and electrochemotherapy.7 Notably, Swiss physicians should offer advanced cSCC patients to be treated in a clinical trial, whenever possible.
IMMUNOTHERAPY WITH IMMUNE CHECKPOINT INHIBITORS
Clinical trials with immune checkpoint inhibitors (ICIs) were designed based on the rationale that expression of cell surface programmed cell death 1 receptor/ligand (PD-1/PD-L1) was linked to poor clinical outcomes in cSCC.17,18 Until the recent introduction of ICIs, there was no approved agent for lacSCC and mcSCC, and available treatments had very limited efficacy with significant adverse reactions.7 The monoclonal PD-1 inhibitor cemiplimab is to date the only approved systemic therapy in cSCC in Switzerland; it is currently indicated for patients with lacSCC or mcSCC who are not candidates for curative surgery or curative radiation (Table 4).7,10 It is important to consider comorbidities for each patient as part of the interdisciplinary team discussions, since some patients, e.g. immunocompromised or post-organ transplantation or patients with autoimmune diseases, were excluded from clinical trials and ICI treatment. Since solid organ transplant recipients (sOTRs) have the highest incidence of SCC, two studies with anti PD-1 have been approved for renal transplant patients with cancer in the U.S. as these patients are eligible for hemodialysis in case of organ rejection. There are more and more case reports about sOTR and anti-PD-1 therapy, and each individual case should be discussed with the interdisciplinary team in case of a high risk of organ rejection with immunotherapy.19–22 Moreover, there are no convincing reasons to date why patients with hematological diseases such as monoclonal gammopathy of undetermined significance (MGUS) or chronic lymphatic leukemia (CLL) should not be treated with PD-1 inhibitors. Pembrolizumab, another PD-1 inhibitor, is currently being investigated in cSCC clinical studies , and was recently approved for cSCC by the FDA.7 Except for cemiplimab, all other systemic treatments are currently used off-label in Europe as well as in Switzerland.7
EGFR INHIBITORS
The epidermal growth factor receptor (EGFR) plays a key role in the activation of multiple downstream signaling pathways involved in cell proliferation, apoptosis, invasion, angiogenesis and metastasis.23 In squamous cells, EGFR plays an important role in regulating the RAS/MAPK, PI3K/AKT and phospholipase C pathways, and is strongly associated with the development of cSCC.24 EGFR overexpression in cSCC has been reported as 43%,25 and appears also to have prognostic implications associated with lymph node metastasis and progression proportional to the metastatic risk.7,25–27 However, a low frequency of somatic mutations of EGFR (2.5–5%) in cSCC has been found, i.e. anti-EGFR therapy may be suitable for a small subset of cSCC patients with genetic activation of EGFR by mutation or amplification.28,29
The EGFR is highly expressed in many epithelial tumors, including cSCC of the head and neck.30 Two monoclonal EGFR targeting antibodies, cetuximab and panitumumab, have been evaluated in patients with cSCC.31,32 For patients with lacSCC and mcSCC who have failed to respond or are intolerant to immunotherapy, cetuximab may be used as second-line treatment after cemiplimab (first-line), preferably in combination with chemotherapy or radiotherapy (Table 4).7 Efficacy with EGFR inhibitors combined with chemotherapy has been shown in advanced cSCC but data is available mainly from clinical case series, i.e. there are no prospective randomized trials that can provide details about durability of responses. Cetuximab may be preferred as the second-line agent for elderly patients with comorbidities, who may not tolerate chemotherapy.7 Other available targeted EGFR inhibitors include small-molecule receptor tyrosine kinase inhibitors such as erlotinib, gefitinib, and lapatinib.7
CHEMOTHERAPY AND ELECTROCHEMOTHERAPY
Although there are no systemic chemotherapies approved for treating advanced cSCC patients, platinum-based agents may be used in the second-line setting when patients fail to respond or are intolerant to anti-PD-1 immunotherapy.7 Chemotherapy in combination with EGFR inhibitors or radiotherapy may be more effective.7 Although electrochemotherapy (ECT) is often available in most centers, there are no prospective, randomized studies about its long-term effectiveness in advanced cSCC. Electrochemotherapy may be considered by the interdisciplinary tumor board and reserved for a very select number of patients, treated at specialized Swiss centers (Table 4).7
SWISS RECOMMENDATIONS FOR USE OF SYSTEMIC THERAPIES
PD-1 INHIBITORS
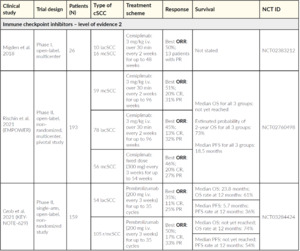
Checkpoint antibody inhibitors such as anti-PD-1 agents function as tumor suppressing factors via modulation of immune cell to tumor cell interaction.33 Cemiplimab, an intravenous human monoclonal antibody directed against PD-1, blocks T-cell inactivation and enhances the immune system’s anti-tumor response. The FDA approved cemiplimab in 201811 closely followed by the EMA in 201910 for treating lacSCC or distant metastatic disease in which curative surgery or radiotherapy is not feasible. Swissmedic approved cemiplimab in May 2020. Initial approval was based on the results of two clinical trials (NCT02383212 and NCT02760498; Table 5).34,35 Migden et al. (2018) reported integrated data from an open-label, multicenter phase I cemiplimab study that included an expansion cohort of 26 lacSCC and mcSCC patients, as well as a nonrandomized global phase II study of 59 mcSCC patients.34 In phase I and phase II, cemiplimab was administered intravenously at 3 mg/kg every 2 weeks for up to 48 weeks (phase I) or 96 weeks (phase II).34 The objective response rate (ORR) was 50% (95% CI: 30–70) and 47% (95% CI: 34–61) for phase I and phase II cohorts, respectively.34 Among responders with a median follow-up of 7.9 months, 61% of mcSCC patients in the phase II cohort had durable disease control with an acceptable safety profile, meaning that they avoided progressive disease for at least 105 days.34 The results of the pivotal single-arm phase II cemiplimab study, also reported by Migden et al. (2020), enrolled a total of 78 lacSCC patients without nodal or distant metastasis from 25 outpatient clinics across Australia, Germany, and the U.S. between June 14, 2016, and April 25, 2018.35 Patients received cemiplimab 3 mg/kg every 2 weeks for up to 96 weeks. The ORR (primary endpoint) was observed in 34 of 78 patients (44%; 95% CI: 32–55), with complete response (CR) seen in 10 patients (13%).35 Stable disease (SD) was observed in an additional 28 patients (36%).35 Median duration of follow-up was 9.3 months and median duration of response had not been reached at data cut-off (October 10, 2018). Estimated 12-month progression-free survival was 58% and estimated 12-month overall survival was 93%.35 Cemiplimab showed an acceptable safety profile, with Grade 3–4 treatment-emergent adverse events occurring in 44% of patients and serious treatment-emergent adverse events occurring in 29% of patients.35 The importance of anti-PD-1 therapy has been further supported by data on treatment with pembrolizumab. The multicenter, open-label, non-randomized phase II EMPOWER-cSCC-1 trial included 193 advanced cSCC patients with a median age of 72 years. At the American Society of Clinical Oncology 2020 (ASCO20) Virtual Congress, the ~1-year follow-up from this large prospective study in advanced cSCC was presented. The results showed that patients treated with cemiplimab demonstrated an objective response rate (ORR) of 46.1%. Among patients who had received prior systemic anti-cancer therapy, ORR was 41.5% and 48.4% in those who had not.36 Results from the post-hoc analysis of phase II trial presented at the ASCO20 Virtual Congress further showed that improvement in global health status/health-related quality of life (HRQL) was observed as early as cycle 3 with clinically meaningful benefit through cycle 12 in advanced cSCC patients treated with cemiplimab.37
Results of the phase II CARSKIN study with pembrolizumab on 39 patients with unresectable cSCC, with no prior systemic treatment and a median age of 80 years, showed a response rate of 38.5% and a median progression-free survival of 8.4 months (NCT02883556).38 Additionally, in KEYNOTE-629 (NCT03284424), the efficacy and safety of pembrolizumab is being evaluated in adults with recurrent/mcSCC or lacSCC.12,39
Use of anti-PD-1 agents in the adjuvant setting are not covered in these Swiss recommendations since clinical trials are ongoing.40–42
Although there are no evidence-based data on when to cease the treatment with the anti-PD-1 antibodies, we suggest similar application as for melanoma.
For metabolic CR (mCR)/CR, patients should be treated for 6 months after CR has been achieved, and for metabolic partial response (mPR)/PR, patients should be treated for 2 years.
EGFR INHIBITORS
Cetuximab is a humanized monoclonal antibody directed against the extracellular domain of EGFR, and is approved in Europe for the treatment of patients with lacSCC of the head and neck in combination with radiation therapy and patients with recurrent/mcSCC in combination with platinum-based chemotherapy.24,43 Off-label use has included cetuximab monotherapy44–46 or cetuximab combined with radiotherapy or cisplatin,45,47–51 for advanced cSCC in a small number of patients in prospective studies or patient cases. Hence, there is a paucity of data with EGFR inhibitors in Europe as well as in Swiss clinical practice. In advanced cSCC (lacSCC and mcSCC patients), first-line cetuximab monotherapy demonstrated a disease control rate (DCR) of 69% at 6 weeks (Table 4).31 In this phase II clinical trial, cetuximab also showed an ORR of 28% and a median progression-free survival (PFS) of 4.1 months with less toxicity in patients with lacSCC and mcSCC.31 Smaller prospective studies and patient cases have shown that higher ORR could be achieved when cetuximab is combined with chemotherapy and/or radiotherapy. In most cases, however, median PFS still remained short (Table 5).45,47,48,52 Cetuximab is recommended in Europe and Switzerland as a second-line treatment after first-line cemiplimab, combined with chemotherapy or radiotherapy. In a single-arm study of 16 patients with advanced cSCC, panitumumab, another anti-EGFR agent, showed similar efficacy (ORR of 31% with 19% partial response (PR), 12% CR).53
CHEMOTHERAPY AND ELECTROCHEMOTHERAPY
No systemic chemotherapies have been approved in Switzerland to date for patients with advanced cSCC (level of evidence 3–4).7 In Europe, platinum agents (i.e. cisplatin or carboplatin), 5-fluorouracil, capecitabine, taxanes, bleomycin, methotrexate, adriamycin, doxorubicin, gemcitabine and ifosfamide have been used off-label either as monotherapy or polychemotherapy for advanced cSCC.7,45 Evidence to date suggests that polychemotherapies are more effective than monotherapies,7 with most responses being short-lived, followed by rapid recurrence, and failing to provide a curative effect.45 In a systematic review of 60 mcSCC cases treated with cisplatin reported by Trodello et al. (2017), a CR was described in 22% of patient cases and PR in 23%, resulting in an overall response of 45%.45 Median disease-free survival for patients who attained CR was 14.6 months.45
ECT is a combination treatment used to reduce tumor progression in which a cytotoxic agent (usually bleomycin or cisplatin) is intravenously injected, followed by pulse application of an electric field into the cSCC tumor mass for enhanced drug delivery to cells.7,54 It has the advantage of high local tumor and bleeding control with minimal damage to normal tissue.7,55 In a European multicenter prospective study of the effectiveness of ECT in the treatment of skin cancer of the head and neck (EURECA), better responses with small lesions (≤3 cm), primary tumors, and naïve tumors (p<0.05; level of evidence 3–4) were reported.55 At 2-months follow-up, CR was achieved in 55% of cSCC, PR in 24%, SD in 15%, and progression in 4%.55 Bertino et al. (2016) concluded that ECT is an effective option for patients with head and neck cSCC when previous treatments had either failed or were not deemed suitable or declined by the patient.55 Overall, ECT was well tolerated and led to a significant improvement of quality of life for patients in this study.7,55
REFLECTION ON CURRENT EU TREATMENT GUIDELINES
Despite the approval of a new systemic treatment option for adult patients with metastatic or locally advanced cSCC who are not candidates for curative surgery or curative radiation in the EU,10 most EU national consensus management guidelines do not yet fully reflect recent evidence and the first-line change in systemic treatment for cSCC. In addition, it is not always clear when to use systemic therapies in lacSCC or mcSCC despite clear margins. Only the European Association of Dermato-Oncology (EADO), European Dermatology Forum (EDF) and European Organization for Research and Treatment of Cancer (EORTC) consensus guidelines (2020) provide a Grade A recommendation for first-line PD-1 inhibition with cemiplimab for patients with lacSCC or mcSCC who are not candidates for curative surgery or curative radiation.7 The 2019 German S3 guidelines for advanced cSCC patients were published prior to EMA approval of cemiplimab and therefore do not recommend any systemic treatment, except in the context of clinically controlled trials.56 The use of PD-1 inhibitors is mentioned as a novel therapeutic approach for inoperable cSCC, and initial data for cemiplimab is noted.9 Clinical trials underway with both cemiplimab and pembrolizumab are also mentioned in the German S3 guidelines.56 The National Comprehensive Cancer Network (NCCN Guidelines®) guidelines for the treatment of advanced cSCC were published a few months after the EMA approval of the first PD-1 inhibitor for the treatment of advanced cSCC.8 Systemic treatment for lacSCC and most cases of mcSCC is not recommended, but in an adapted footnote, immunotherapy (cemiplimab or clinical trial) should be considered if curative radiotherapy and curative surgery are not feasible.8
CONCLUSIONS
The recommendations herein are aimed to provide guidance to Swiss clinicians with the most up-to-date recommendations on how immunotherapy and other systemic therapies can be integrated into the treatment algorithm for advanced cSCC. In summary, an interdisciplinary approach is mandatory for patients with advanced cSCC to optimally manage their disease in the long-term.7–9 Treatment for primary low-risk cSCC remains surgical excision with post-operative margin assessment or Mohs micrographic surgery. Radiotherapy should be considered as curative treatment for inoperable primary common cSCC or for non-surgical candidates. Systemic therapy for advanced cSCC should be offered, whenever possible, as part of a clinical trial. In Switzerland, anti-PD-1 antibodies (i.e. cemiplimab) should now be the first-line systemic treatment for patients with mcSCC or lacSCC who are not candidates for curative surgery or radiation. Whilst there is clear agreement about the place of approved PD-1 inhibitors as the first-line systemic treatment, second-line treatment options are less clear. Cetuximab as well as chemotherapeutic agents may be discussed by the interdisciplinary tumor board as second-line treatment options for patients with advanced cSCC. Electrochemotherapy (ECT) may also be considered by the interdisciplinary tumor board and reserved for a very select number of patients in a few specialized centers. Best supportive care should be offered to patients with advanced disease to optimize symptom management and improve quality of life, and the frequency of follow-up visits and investigations for subsequent new cSCC should be determined by underlying risk characteristics.
The authors of these Swiss recommendations identified several outstanding questions that still need to be addressed in this field in the coming years: (1) how should PD-1 inhibitors be used in immunocompromised patients? (2) Can relative risks be reduced further with combination regimens? and (3) how effective is immunotherapy for advanced cSCC in the adjuvant and neoadjuvant setting?7–9,57 Notably, ICIs are not recommended in the adjuvant/neoadjuvant setting outside of clinical trials since there is no data available; clinical trials are ongoing. In addition, several gaps have been identified in the existing EU guidelines for immunotherapy usage in cSCC, including limited use of checkpoint inhibitors in advanced cSCC patients taking immunosuppressive medications (e.g., organ transplant recipients, autoimmune disease).7–9,57 There is also no comprehensive information for systemic cSCC therapies in patients with underlying hematologic malignancies such as chronic lymphocytic leukemia (CLL). In early stage cSCC, there is currently no robust evidence to support the use of adjuvant or neoadjuvant systemic treatment for cSCC. It is, however, feasible that results from ongoing studies with the anti-PD-1 monoclonal antibodies, cemiplimab and pembrolizumab, may address some of these treatment gaps.
If patients decide against a therapy, our tasks include providing precise information about the tumor growth, expected and possible pain of the invasive bleeding tumor, as well as networking within a palliative service.
TAKE-HOME MESSAGES
The development and approval of the programmed-death protein 1 (PD-1) inhibitor cemiplimab has led to a new era in the systemic treatment of advanced cutaneous squamous cell carcinoma (cSCC). These Swiss recommendations provide guidance for the management of patients with lacSCC or mcSCC and will also help Swiss physicians in their decision-making.
-
Systemic therapies should be considered for patients with advanced cSCC following an interdisciplinary tumor board discussion, and offered as part of a clinical trial whenever possible.
-
In Switzerland, the anti-PD-1 monoclonal antibody cemiplimab is recommended as the first-line systemic treatment for patients with distant metastatic or locally advanced cSCC who are not candidates for curative surgery or radiation.
-
Ongoing clinical studies with immune checkpoint inhibitors will help to answer several critical questions involving the usage of anti-PD-1 antibodies in locally advanced and distant metastatic cSCC patients.
CONFLICT OF INTEREST (COI)
The authors declare that they hold advisory relationships with Sanofi that could be considered a potential conflict of interest related to the submitted manuscript.
ACKNOWLEDGEMENT
We thank Dr Ellen Heitlinger, H+O communications Ltd., Zurich, Switzerland for her medical writing support of the manuscript (including writing, language editing, referencing, formatting, and proofreading), which was financially supported by Sanofi-Aventis (Switzerland) AG. Sanofi-Aventis did not have any decision-making role in the development of the manuscript and did not influence its content in any way.
Author Contributions
All authors contributed to and approved the final manuscript.