The microbiome and its importance in cancer
The human microbiome comprises the bacteria, viruses, fungi, protozoa, and other microbes that live throughout the human body, along with their collective genomes and metabolic products.1 An average human body is made up of 30 trillion cells, but also contains about 39 trillion microbial cells. In addition, there are about 25,000 human genes, but 2 million to 20 million microbial genes.2 Altogether, the human microbiota contribute to approximately 1–3% of the body mass.
Microorganisms colonize various anatomical sites, such as the skin, the mucosa, the gastrointestinal, respiratory, and urogenital tract, as well as the mammary glands.3 The gastrointestinal tract hosts most of this bacterial microbiota, with the colorectum having the highest density, of up to 1011 bacterial cells/ml of colon material.4 The mammalian immune system is a complex network of innate and adaptive components throughout all tissues that have a crucial role in host defense against a variety of potentially harmful external agents, as well as monitoring of endogenous homeostatic perturbations.
The gut microbiome can be disturbed by environmental incursions (e.g., antibiotic use, changes in diet or geography), impairment of the host-microbiome interfaces, or alterations in the immune system.5 This can result in systemic dissemination of commensal microorganisms, increased vulnerability to pathogen invasion, and aberrant immune responses. Globally, infectious agents or infection-associated chronic inflammation are believed to be responsible for about 16% of human cancers, with higher rates in less developed countries (22.9%) than in more developed countries (7.4%).6
Non-commensal bacteria and viruses are well-known contributors to tumorigenesis of solid tumors as demonstrated for Epstein-Barr virus in nasopharyngeal cancer and different lymphomas, human papillomavirus in cervical and oropharyngeal cancer, and Helicobacter pylori in gastric cancer. Interactions between the microbiome and the immune system participate in the regulation of infections and commensal dissemination and have been linked to a number of non-communicable gastrointestinal diseases. These include inflammatory bowel disease (IBD) and celiac disease, as well as rheumatoid arthritis, metabolic syndrome, neurodegenerative disorders, and cancer.7–12
Association of nutrition, lifestyle, gender, and microbiome composition
There are around 3.8 ×1013 million microorganisms in the human intestine. They are known to influence basic functions like metabolism, nutrition, immunomodulation, and pathogen tolerance, to help maintaining the physiology and health of their host.13 A “rural” diet is enriched in dietary fibers with anti-inflammatory-like microbiota predominantly with bacteria that produce key metabolites, including short-chain fatty acids. On the contrary, an “urban” diet that is rich in animal fat and protein induces a proinflammatory microenvironment, which can lead to increased predisposition to diseases like colorectal cancer (CRC).
The composition and function of the gut microbiome are shaped by dietary timing and changes in dietary patterns.14 Gut dysbiosis is characterized by an imbalance in the synthesis of microbial antigens and metabolites by commensal and pathogenic bacteria. The immune system and the gut microbiome work together to maintain the gut in a state of homeostasis, whereby changes in the gut microbiome composition can trigger immune dysregulation, which can promote, in turn, chronic inflammation and tumor development (Figure 1).
Gut microorganisms and their toxic metabolites can use the circulatory system to disseminate throughout the body. This can result in an imbalance in the physiological status of the host, and the secretion of various neuroactive molecules through the gut-brain, gut-liver, and gut-lung axes, induce inflammation and subsequently foster tumorigenesis at specific organ sites.15 As a result, the gut microbiota and their metabolites might be used to identify an elevated risk for certain tumors, and might lead to novel insights into their pathogenesis as well as potential therapeutic and preventive strategies.
Such predictions can be facilitated by longitudinal studies that monitor the fecal and oral microbiomes, the diet and lifestyle in large diverse patient populations. The establishment of reproducible microbiome signatures might allow the precise stratification of an individual’s cancer risk or prognosis (Figure 2) and could be used in combination with other well-known risk factors. In addition to preliminary dietary and lifestyle improvements, modulation of the microbiota might be used to prevent the colonization of pathogenic or cancer-supporting microbial communities in patients with increased risk of cancer. Finally, treatment can be improved; after a patient has been diagnosed with cancer, direct microbial modifications might be used to eliminate oncogenic and toxic drug metabolites responsible for adverse events, disease progression or resistance (Figure 2).
Microbial colonization of the human body begins at birth, and then over the next 2 to 3 years the gut microbiota develops a series of taxonomic peaks and troughs until it reaches its adult-like richness and proportions.16 The diversity, abundance, and role of the early life gut microbiota are influenced by a number of factors, such as gestational age, delivery mode, birth weight, feeding modes, antibiotic intake, the maternal microbiome, and diet. According to several studies, dysbiosis of the gut microbiota in early life has been linked to a variety of childhood and adult diseases and their outcomes, including asthma, atopic dermatitis, diabetes, allergies, obesity, cardiovascular disease, and neurological disorders.17–20
A recent animal study provided evidence that the microbiome can even function as a mediator of negative consequences of an impaired health status from one generation to the other. Indeed, maternal obesity has been linked to cognitive and sociality decline in their children. In addition, the microbiota-short-chain fatty acid-brain axis is known to mediate obesity-induced maternal behavioral disorders.21 Dietary modification of the maternal microbiome through introduction of a high-fiber diet during pregnancy can abrogate this behavioral and cognitive malfunction.21 Similar effects have been shown for the immune and metabolic systems of the offspring.22 A recent study has also provided valuable insights into gut microbes from the time before industrialization through DNA sequencing of the microbiomes of 1,000–2,000-year-old human stool samples.23
Almost a decade ago, Flak et al. (2013) introduced the concept of the “microgenderome” to describe the sex-specific differences in the male and female microbiome.24–26 It was further characterized by describing the bidirectional interactions between the microbiota, hormones, immunity, and disease susceptibility. This is probably due to the differential sex hormone levels in males and females, but also affected by the microbiome itself. Sex differences in the microbiota composition drive the differences in both innate and adaptive immunity, and the sex-differential innate and adaptive immune systems, in turn, drive the sex differences in the microbiota composition.27
This microgenderome is known to have an important role in the bidirectional communication through the gut-brain axis, which further influences immunity, metabolism, neurodevelopment, and behavior.28 As a first step toward personalized precision medicine, a deeper understanding of the fundamental processes that control sex-specific differences in immune responses and their relation to the microgenderome is required to enable improved preventive and therapeutic strategies for men and women.29
The microbiome and disease
Clostridium difficile infection remains a continuously evolving global problem that is caused by this Gram-positive, spore-forming, toxin-producing anaerobic bacillus that is transmitted in humans through the fecal-oral route.30 This bacterium colonizes the large intestine and produces two protein exotoxins that can cause colitis in a susceptible population: the TcdA and TcdB exotoxins. The most important risk factor for C. difficile infection remains the use of antibiotics such as ampicillin, amoxicillin, cephalosporins, clindamycin, and fluoroquinolones.31 One effective treatment of this major health problem has been fecal microbiota transplantation (FMT).31
Candidiasis is a major fungal infection due to antibiotic-induced alterations in the gut microbiome. This is caused by Candida, a normal constituent on the body and in the mouth, gastrointestinal tract, and vagina.32 Infections due to Candida spp. are widely recognized as significant causes of morbidity and mortality in the healthcare setting.33 Any change in the immune defense system and microbiome environment of the body can result in localized infections or more serious systemic infections, depending on the general health of the patient. The five most common Candida pathogens cause more than 90% of these invasive diseases: C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei.33
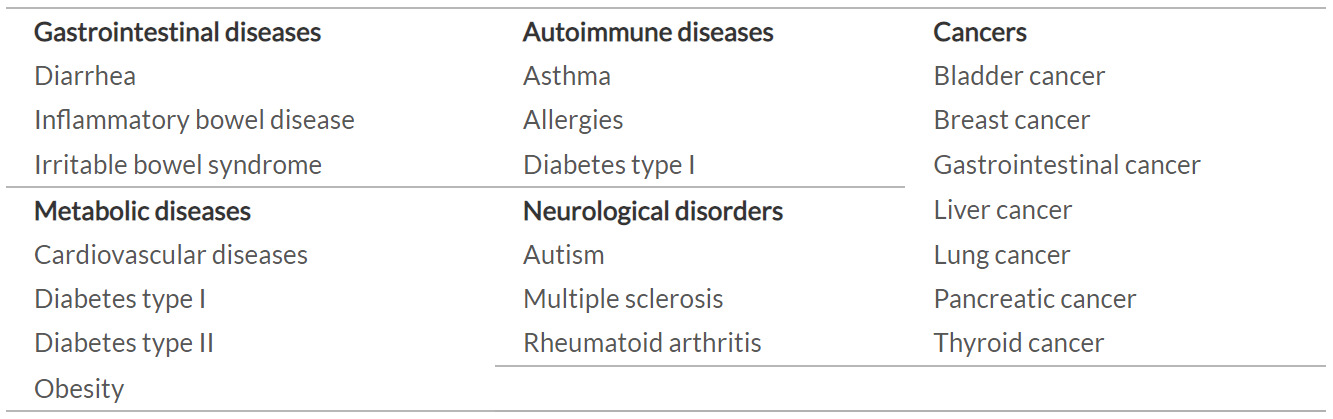
The human gut microbiome is known to have a role in the etiology of various diseases, including behavioral disorders, IBD, obesity, type II diabetes mellitus, cardiovascular disease, atopic asthma, autoimmunity, and cancers (Table 1).34 Various types of IBD have been linked to changes in the structure and function of the bacterial and fungal gut microbiota, including Crohn’s disease and ulcerative colitis.35,36
Early life environmental exposure has been associated with increased risk of childhood asthma in a variety of epidemiological studies. Cesarean birth, antimicrobial administration, formula feeding, exposure to furred pets, and environmental pollutants are all known to shape the developing gut microbiome.55–58 Infants at risk of developing asthma later in life have been shown to have early life microbiota dysbiosis. In infants at risk of developing atopy or asthma, observations from separate geographically distinct birth cohorts that encompassed different socioeconomic and racial groups have regularly reported depletion of symbiotic bacterial taxa such as Faecalibacterium, Akkermansia, and Lachnospira.
The gut microbiota has recently emerged as a crucial mediator in biochemical signaling between the gastrointestinal tract and the central nervous system; i.e., the gut-brain axis.59 A number of behavioral disorders have been associated with perturbed enteric microbiota, including autism spectrum disorder in children.60,61 Furthermore, obesity and type II diabetes mellitus have been associated with dysbiotic gut microbiota and a substantially reduced bacterial complexity.62 In addition, obesity has also been reported to be associated with a decline in the relative abundance of Bacteroides spp. The microbial metabolism of dietary choline and carnitine, which are abundant in the Western diet, has been linked to increased risk of cardiovascular disease.63 Trimethylamine is generated by the metabolism of these compounds, and it is oxidized in the liver to trimethylamine-N-oxide, an amine oxide that is linked to the development of atherosclerosis.
Perturbations in the gut microbiota have also been reported in patients with autoimmune disorders, such as rheumatoid arthritis and multiple sclerosis. The enteric population of Prevotella copri expands in patients with rheumatoid arthritis, and the autoantigens in these patients have high homology to Prevotella-associated peptides.64,65 The relative abundance of Bacteroidetes that produce short-chain fatty acids was inversely correlated with systemic inflammatory markers in pediatric patients with multiple sclerosis, while bacterial microbiota richness was positively associated with circulating pro-inflammatory Th17 cells.66 Changes in the lung microbiota in another progressive inflammatory disease of the lung, chronic obstructive pulmonary disease, have also been linked to exacerbations and to inflammatory responses in the host.67
Gastric cancer is the fourth leading cause of cancer deaths worldwide, as it accounts for nearly 769,000 deaths each year.68 Helicobacter pylori is a well-known risk factor for gastric cancer.69 More than half of the population worldwide is infected with H. pylori, which can alter the gastric microbiome profile and cause H. pylori-related diseases through modulation of the stomach acidity. In addition, gastric mucosa-associated lymphoid tissue lymphoma (MALT) is also known to be associated with H. pylori infection.70 In most cases, H. pylori eradication therapy should sufficient to treat MALT lymphomas, according to updated European Association for Medical Oncology (ESMO) clinical practice guidelines.71
Colorectal cancer is a heterogeneous disease of the intestinal epithelium that is characterized by the accumulation of mutations and by dysregulated immune responses. It is the second leading cause of cancer-related deaths globally, with increasing incidence, especially in individuals under 50 years of age.72 Here, IBD, obesity, diabetes, and a high-fat, high-protein diet have all been related to intestinal dysbiosis, and are all known to be risk factors for CRC.73 Indeed, patients with CRC have a different taxonomic composition compared to healthy individuals. In CRC tumor tissue, a higher relative abundance of putatively pro-carcinogenic bacteria has been observed, including Fusobacterium nucleatum, Escherichia coli, Bacteroides fragilis, Enterococcus faecalis, Streptococcus gallolyticus, and Peptostreptococcus spp.. In contrast, the so-called defensive genera are reduced, such as Roseburia, Clostridium, Faecalibacterium and Bifidobacterium.38,74
Lung cancer is by far the most common cause of cancer deaths in both men and women, and it accounts for about a quarter of all cancer deaths.75 The composition of the lung microbiota is strongly associated with lifestyle, tobacco smoke, and environmental and genetic risk factors.76 Dysbiosis of the lung microbiota can influence the risk of cancer at multiple levels, including through chronic inflammation and oncogenes. In patients with lung cancer, a substantial enrichment of the genera Granulicatella, Abiotrophia, and Streptococcus has been shown, which was associated with a decline in the population diversity compared to stable controls.77 Liu et al. (2019) reported that the lung-cancer-associated microbiota is enriched in Streptococcus but deficient in Staphylococcus, which implies that Streptococcus is harmful and Staphylococcus is protective in the development of lung cancer.45
Harnessing the microbiome for cancer treatment and prevention
Fecal microbiota transplantation as a technique was first reported in 1958, and it is now widely recognized as a safe and effective treatment for recurrent C. difficile infection.78 The phyla Bacteroidetes and Firmicutes are considered to be key components of the material to be transplanted. More than 90% of patients with recurrent C. difficile infection can be successfully treated with oral or rectal transplant of feces from a stable pretested donor, combined with simultaneous cessation of all antibiotic use in the recipient.31 The results of a randomized controlled trial on FMT demonstrated that a combination of vancomycin and a nasoduodenal tube infusion of donor feces was found to be safer and more effective than vancomycin alone. However, there have not been many studies on FMT to date, and although case series have shown promise, further research is required to fully understand the potential role of FMT in primary C. difficile infections.79 In addition, efforts are underway to produce a suitable mixture of cultured fecal bacteria to use for stool replacement in FMT.31 Baruch et al. (2021) and Davar et al.(2021) reported the first-in-human clinical trials to determine whether FMT impacts the response of metastatic melanoma patients to anti-programmed cell death-1 (PD-1) immunotherapy.80,81 These studies provided proof-of-concept data for the influence of FMT on immunotherapy responses in patients with cancer. However, identification of the ideal donor through microbiological typing of stool samples is currently challenging.82 A thorough understanding of the role of the intestinal microbiota in each chronic disease is required prior to development any personalized treatment strategy using FMT.82
The eradication of H. pylori infection is an effective strategy for lowering the risk of gastric cancer. Clarithromycin-containing proton-pump inhibitor (PPI) triple therapy was recommended as first-line treatment for H. pylori infection by the Maastricht V/Florence Consensus Study.83 Antibiotic treatments of H. pylori infection change the composition of the gastric microbiota, according to many reports.84 Some probiotics minimize fluctuations in the gut microbiome profile, reduce antibiotic-induced side effects, and increase H. pylori eradication rates. Lactobacillus spp. are commonly used in food processing and clinical practice to regulate the microbial environment in the human gastrointestinal tract; indeed, these are one of the most well-studied probiotics.85 Multi-strain probiotics have also been shown to improve H. pylori eradication rates and avoid adverse events.86 A probiotic mixture that contained Lactobacillus and Bifidobacterium was shown to have beneficial effects against H. pylori, with low incidence of side effects. In patients with gastric cancer and a prior gastrectomy, a mixture of Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus improved host immunity and reduced inflammation.87
The targets of the immune checkpoint inhibitors (ICIs) include cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), PD-1, and programmed cell death-ligand 1 (PD-L1). ICIs are increasingly used in daily clinical practice to improve clinical outcomes for patients with advanced non–small cell lung carcinoma (NSCLC), renal cell carcinoma, urothelial carcinoma, melanoma, and squamous cell carcinoma of the head and neck.88 Preclinical models have demonstrated that the composition of the gut microbiota and its modification in mouse models can alter the efficacy of ICIs and the incidence of immune-related adverse events.89,90 Several studies have shown asignificant association between the commensal microbial composition and clinical response. Matson et al. (2018) reported that the most abundant species in responder patients with melanoma included Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium.91 Furthermore, Routy et al. (2018) showed an association between clinical responses to ICIsand the relative abundance of Akkermansia muciniphila in patients with NSCLC and renal cell carcinoma.92 Gopalakrishnan et al. (2018) reported a relative abundance of bacteria of the Ruminococcaceae family in responder patients with melanoma.12 Chaput et al. (2017) observed improved survival outcomes for patients with melanoma, whose gut microbiota included Faecalibacterium genii and other Firmicutes.93 In a prospective study, Frankel et al. (2017) reported that the commensal flora can vary in responders depending on the ICI used.94 In nivolumab-ipilimumab responders, the gut microbiota was enriched with Faecalibacterium prausnitzii, Bacteroides thetaiotaomicron, and Holdemania filiformis. In patients who were responders to pembrolizumab, the gut microbiota was enriched with Dorea formicigenerans.
The human gut microbiome can also be affected by co-medications, such as the use of antibiotics and PPIs in cancer patients treated with ICIs.95 It has been reported that the use of antibiotics can reduce the efficacy of ICIs and affect the outcomes in patients, who receive ICIs for cancer treatment.96,97 Additionally, antibiotic use is associated with a lower overall response rate as well as shorter progression-free survival, and overall survival, irrespective of the cancer type. Therefore, antibiotics must be avoided before and during treatment with ICIs, due to their negative effects on key outcome parameters.95 After initial results of individual studies have been conflicting (positive, negative and no association), a recent meta-analysis of concurrent ICI and PPI therapies did show no associated with overall survival or progression-free survival.1,88,96,97 Further research into the effects of co-medications on particular ICIs in patients with specific cancer subtypes will be required to develop reliable recommendations.
In patients with melanoma, a correlation between the gut microbiota composition and the incidence of ICI-mediated colitis has been reported.90,92,93 Firmicutes were shown in abundance in stool samples from patients with immune-mediated colitis. In ICI-treated patients, however, an excess of Bacteroidetes was linked to low incidence of colitis.90,93 In the future, FMT might represent an option for the treatment of high-grade immune-related colitis, if the efficacy and safety profiles of this treatment prove to be more beneficial than the current therapies.95 Recently, a single-arm clinical trial investigated the safety and efficacy of FMT obtained from individual long-term responder melanoma patients together with anti-PD-1 therapy in PD-1–refractory metastatic melanoma patients.81 These data showed that single colonoscopy FMT administration together with PD-1 blockade successfully colonized the gut of responders and reprogrammed the tumor microenvironment to overcome primary resistance to anti-PD-1 therapy in patients in this setting. In patients with PD-1–refractory melanoma with immunological potential to respond to treatment but an unfavorable microbiota composition, FMT altered the microbiome composition toward taxa supporting the anti-PD-1 efficacy, to induce clinical responses to the anti–PD-1 therapy.81
Tumor neoantigens represent a mechanistic prerequisite for effective cancer immunotherapy.98 A collection of cancer-specific molecular aberrations that are commonly known as the “tumor neoantigenome” represents an ideal target for cell-mediated immunity and cancer immunotherapy, with the highest possible specificity for cancer considering the natural selectivity of cytotoxic, adaptive immune cells.99,100 While tumor neoantigens can distinguish cancer cells from healthy cells, epitopes developed by gut and extra-gut microbial species as part of their natural gene expression programs can resemble tumor neoantigens, a phenomenon that is known as molecular mimicry. Molecular mimicry of tumor neoantigens by microbial species is likely influencing the host anti-cancer immune response through neoantigen-reactive-T cells, which might explain, why some patients respond well to immune checkpoint blockade while others do not. Immunological mimicry of tumor neoantigens by microbial peptide products is a possible reason for the functional importance of the gut microbiome in checkpoint immunotherapy responsiveness.101–103
Although the outcomes of allogeneic hematopoietic stem-cell transplantation (allo-HSCT) have improved over the last decade, infections and graft-versus-host disease (GvHD)remain two of the most common complications, and these contribute significantly to early transplant-related mortality.104 The intestinal microbiome is disrupted during allo-HSCT in a multifaceted mechanism that involves numerous factors, such as conditioning, diet, and antibiotic treatment. FMT has recently emerged as a possible intervention to aid microbiome recovery and potentially intervene in this interplay. Although, adding beneficial bacteria or their metabolites to animal allo-HSCT models has been shown to reduce GvHD, there are still many questions that still need to be answered about the role of the intestinal microbiota in human allo-HSCT.105
Conclusions
-
The human microbiome has a significant role in the health of the host and is involved in the development of a wide range of diseases.
-
Substantial changes in the abundance of specific bacteria can be detected in patients with cancer. These might serve as biomarkers for screening, prediction of treatment responses and prognosis.
-
Modulation of the gut microbiome is a promising strategy to enhance treatment efficacy and reduce adverse effects of many therapies.
-
Future research should investigate ways to modulate the gut microbiota and explore its short-term and long-term benefits through clinical trials.
CONFLICT OF INTEREST
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The author crafted and approved the final manuscript.