INTRODUCTION
Salvage therapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT) is considered as the treatment of choice for patients with classical Hodgkin’s lymphoma (cHL), who are unresponsive to or relapse after frontline chemotherapy.5–10 However, after a second relapse the prognosis after ASCT is usually poor.11,12
In patients with relapsed/refractory cHL, allogeneic stem cell transplant (allo-SCT) has been shown as a feasible treatment option.13 Recently, improved response rates were observed in clinical trials investigating immune checkpoint inhibitors, such as nivolumab and pembrolizumab, as further options in relapsed/refractory cHL patients.14 However, in the majority of patients, these treatment options are non-curative.
Following factors are associated with an increased risk of progression when detected prior to ASCT:
-
Presence of primary refractory cHL, defined as no remission from first-line chemotherapy or disease progression within the first 3 months after the end of treatment1,15
-
Duration of initial remission <12 months1,16
-
Relapse with evidence of extranodal disease1,17
-
Chemoresistance to salvage therapy1,18
-
≥2 lines of treatment and relapses before ASCT19
-
Evidence of vital disease residues at the time of ASCT19
-
Positive 18FDG-PET scan after 1 or 2 lines of salvage therapy prior to ASCT.20
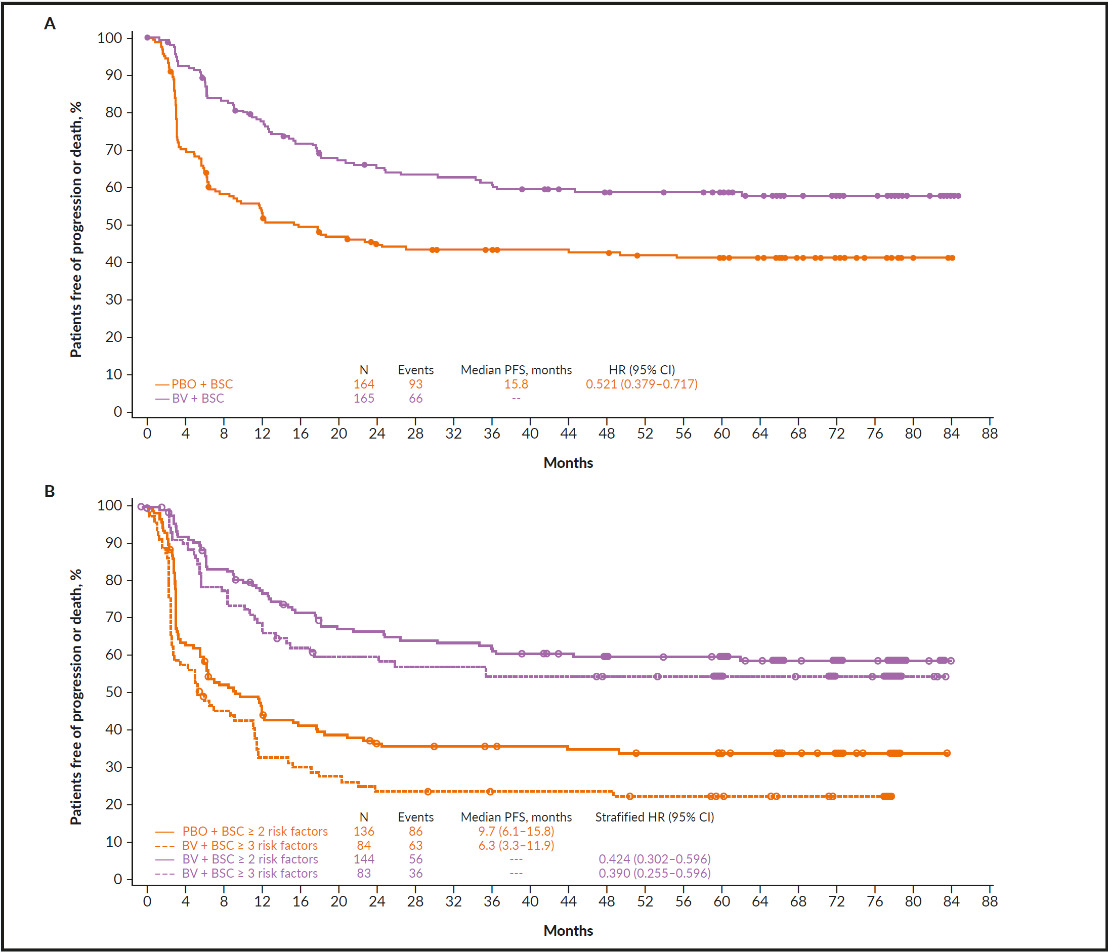
The above-mentioned criteria have been independently confirmed by the AETHERA Study Group in the phase III AETHERA trial, which investigated brentuximab vedotin consolidation after ASCT in patients with cHL at high risk of relapse or progression.1,2 The authors of the study showed that brentuximab vedotin maintenance therapy prolonged the PFS compared with placebo (HR: 0.52), especially in patients with multiple risk factors (HR: 0.42) (Figure 3).1,2,21 Brentuximab vedotin is an antibody-drug conjugate (ADC), which binds to the surface marker CD30 and is highly expressed on Reed-Sternberg cells. The monoclonal antibody is conjugated to monomethyl auristatin E (MMAE), a synthetic neoplastic agent. After binding and internalization of the cytotoxic payload, MMAE is released in a targeted manner.22
Here, we report the case of a female patient with relapsed/refractory cHL, which demonstrates that treatment with brentuximab vedotin is well-tolerated and effective.
CASE PRESENTATION
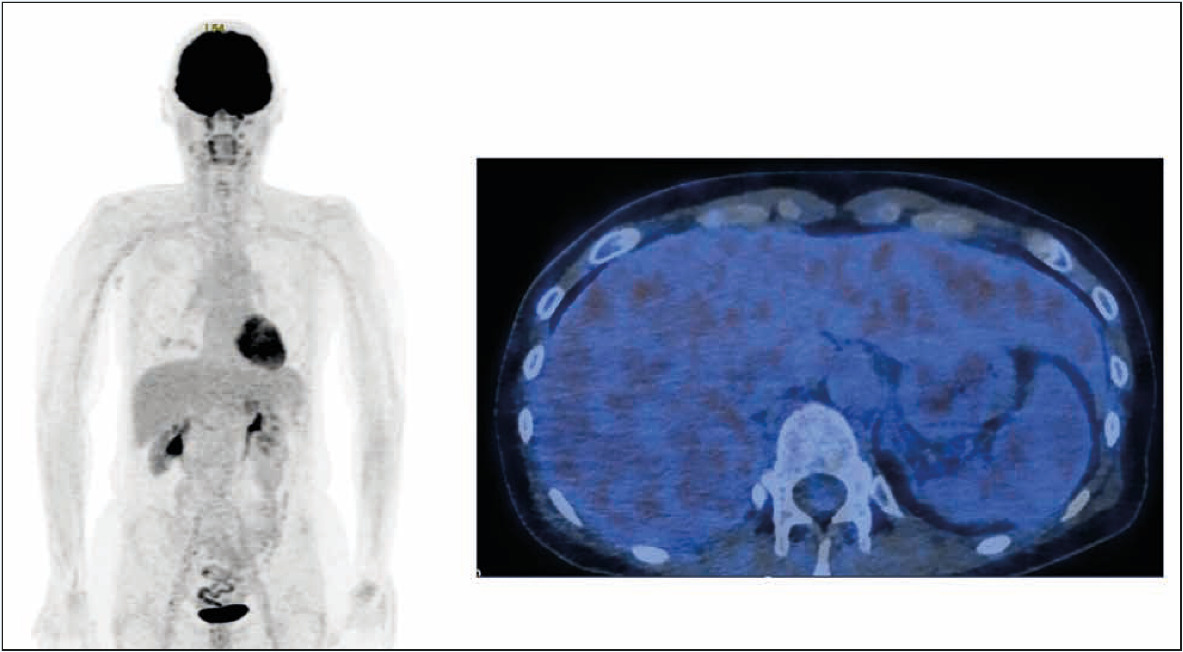
A 49-year old female patient had been suffering from marked pruritus since February 2013. She also started to experience intermittent sweating (sufficient to require her to change her nightdress) in 2014; however, the onset of menopause occurred at the same time. Over the previous year, the patient had lost 4kg. A comprehensive investigation of the symptoms, including a dermatologist examination, remained inconclusive. In April 2015, a computed tomography (CT) scan revealed lesions of unknown origin in the spleen, liver and lungs, giving rise to a suspicion of a paraneoplastic process (Figure 1).
The patient first presented in our department at the Triemli City Hospital in April 2015. The pathological lesions were first investigated by bronchoscopy, which did not reveal any abnormalities, and mediastinoscopy, which showed enlarged paratracheal lymph nodes. Due to the procedures, the patient experienced hemothorax that required several pleural punctures, with a thoracoscopy ultimately performed to evacuate the consolidated residual hematoma. Histopathology revealed a diagnosis of classic Hodgkin’s lymphoma of mixed type, with positivity for CD15 and CD30. A PET/CT scan showed advanced stage IVB disease, with lymphadenopathy (cervical, supraclavicular, bilateral mediastinal and peribronchial, mesenteric, right external iliac, and splenic) and extranodal (bilateral multifocal pulmonary, hepatic, and bone marrow) involvement.
FIRST-LINE TREATMENT: DOXORUBICIN, BLEOMYCIN, VINBLASTINE AND DECARBAZINE (ABVD) POLYCHEMOTHERAPY
Despite the advanced stage, there was strong curative potential in this case. The combination of adriblastina, bleomycin, vinblastine and dacarbazine (ABVD) was selected as it promised better tolerability and less time off work. In addition, as compared with escalated bleomycin, etoposide, adriblastina, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) therapy, ABVD therapy could be started sooner after the hemothorax.
At the end of May 2015, the patient received a first cycle of ABVD as an outpatient. After 2 cycles, PET/CT imaging revealed complete metabolic response and very good morphological response; the pruritus and night sweats had improved rapidly. In view of the very good clinical and imaging response, all 6 cycles of ABVD were administered until the end of October 2015. Further checkups were unremarkable until the end of March 2016, when the patient presented with inguinal lymph node enlargement. A CT revealed a disseminated relapse of Hodgkin’s lymphoma with hepatic, splenic, lymph node (external iliac), and pulmonary involvement. The right external iliac vein was severely compressed by the surrounding lymph nodes. Histology from a liver biopsy and aspiration of the iliac lymph node confirmed the relapse.
FURTHER TREATMENT WITH CHEMOTHERAPY AND ASCT
Between May and June 2016, the patient received reinduction chemotherapy with dexamethasone, high-dose cytarabine, platinum (cisplatin) (DHAP); due to the extent and aggressiveness of her disease, 3 cycles instead of the 2 usual cycles were administered. Subsequent PET/CT imaging revealed complete metabolic and partial morphological remission. In July 2016, after a successful stem cell harvest, the patient underwent high-dose salvage chemotherapy (carmustine, etoposide, cytarabine, melphalan [BEAM] regimen), followed by ASCT.
In addition to the expected complications of this aggressive treatment, such as neutropenic fever and pronounced mucositis with the need for parenteral nutrition, the patient developed aspergillus pneumonia during the 10-day period of aplasia. This required several months of voriconazole therapy. In September 2016, the patient suffered acute right-dominant shoulder pain with anterior subdeltoid bursitis, resulting in a “frozen shoulder”. This was treated with physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs).
USE OF BRENTUXIMAB VEDOTIN
The use of brentuximab vedotin for maintenance therapy was subsequently discussed. Reasons for this included early relapse (after 5 months) and marked extranodal involvement. In addition, the patient had already experienced multiple complications, and the 30- to 45-day post-ASCT limit specified in the AETHERA study protocol could not be met. Furthermore, after high-dose chemotherapy, the patient was suffering from bilateral grade 1 polyneuropathy in her feet, primarily the left foot.
After consultation with the patient, consolidation therapy with brentuximab vedotin was initiated in January 2017. Although the medicinal product information for brentuximab vedotin recommends that treatment should be started at 1.8mg/kg, the patient initially received a slightly reduced dose of 1.6 mg/kg due to polyneuropathy. At this dose, 7 cycles were administered without complications. Subsequently, the dose was reduced to 1.2 mg/kg for a further 8 cycles due to slightly worsened paresthesia with polyneuropathy up to grade 2 (common terminology criteria for adverse events [CTCAE]: 4.0). Therefore, it was possible to administer a total of 15 out of the 16 planned cycles; the last cycle was omitted due to the increased symptoms in her right foot (with her foot feeling cold and going to sleep).
In terms of the treatment benefit, the patient was able to return to her job on a part-time basis in March 2017. Around two months after the end of treatment, from January 2018, the patient reported that her polyneuropathy had slightly improved to grade 1, which was confirmed at the consultation in April 2018. At the same time, a CT scan showed sustained complete remission, which was confirmed by a PET/CT in April 2019 (Figure 2). Apart from the partially pre-existing polyneuropathy, and mild pruritus not requiring treatment, no adverse reactions were observed under treatment with brentuximab vedotin.
FOLLOW-UP
Follow-up visits are performed in a 3−4-months interval and include clinical assessment, laboratory testing, and flushing her Port-a-Cath. The most recent CT scan confirmed complete remission. At her last clinical follow-up appointment in December 2020, the patient stated that she still experiences only mild polyneuropathy and fatigue (both grade 1). The discomfort associated with the frozen shoulder had improved but not resolved completely. The patient is currently working part-time.
BOTTOM LINE
During therapy with brentuximab vedotin from January to November 2017, the patient was able to return to her job and subsequently to gradually increase her workload. The treatment gave the patient some certainty and she also tolerated it well overall. There were also no issues with adherence to the treatment. Despite the high-risk features and a disease course beset with multiple complications, the patient is still in remission three years after the end of maintenance therapy and has been able to gradually return to her normal life.
In clinical practice, this means that maintenance therapy with brentuximab vedotin should be considered for all patients with Hodgkin’s lymphoma after autologous stem-cell transplantation (ASCT) if they have at least one risk factor for progression. Brentuximab vedotin is a well-tolerated and effective drug for the treatment of Hodgkin’s lymphoma which is likely to be used increasingly and in alternating combinations in the future, including in first-line therapy. In this regard, the results of the GHSG HD21 study, in which various Swiss centers are involved, are eagerly awaited.23
DISCUSSION
AETHERA: BRENTUXIMAB VEDOTIN DELAYS PROGRESSION AFTER ASCT IN HIGH-RISK CHL PATIENTS
The phase III, randomized, double-blind AETHERA trial included 329 patients with cHL who had undergone ASCT after high-dose therapy and were found to have at least one of the following risk factors for progression1:
-
Primary refractory Hodgkin’s lymphoma1
-
Relapse within 12 months after completion of the first-line chemotherapy1
-
Extranodal involvement at the start of pre-ASCT salvage chemotherapy1
Additionally, patients were stratified regarding the following risk factors: complete versus partial remission versus stable disease in response to the most recent salvage therapy, B symptoms at relapse, and ≥2 previous salvage regimen.21 Patients were treated with brentuximab vedotin (1.8 mg/kg) (n=165) or received placebo (n=164) for 16 cycles starting 30−45 days post-stem-cell transplantation. It was found that early consolidation therapy resulted in significantly longer progression-free survival (PFS) (42.9 vs 24.1 months; p=0.0013), while patients with ≥2 risk factors were more likely to achieve even greater clinical benefit from treatment (Figure 3). Treatment was generally well-tolerated. The safety profile of brentuximab vedotin in the AETHERA trial was consistent with the profile known from previous studies.
Further analysis of the data from AETHERA revealed that consolidation therapy had no significant effect on patient’s quality of life (p=0.2127), based on European Quality of Life-5 Dimensions (EQ-5D) scores.24
BRENTUXIMAB VEDOTIN AS A FRONTLINE THERAPY FOR HODGKIN’S LYMPHOMA
For the first-line treatment of cHL patients, brentuximab vedotin in combination with adriblastina, vinblastine and dacarbazine (BV+AVD) offers a good alternative, as demonstrated by the ECHELON‑1 data.3,4 A total of 1,334 patients newly diagnosed with stage III or IV Hodgkin’s lymphoma participated in the phase III open-label study. Study participants were randomized 1:1 to receive either 6 cycles of brentuximab vedotin (1.2 mg/kg intravenously on days 1 and 15) plus AVD (BV+AVD) or 6 cycles of ABVD regimen.3 Treatment with BV+AVD resulted in an improved 2‑year modified PFS rate (including the time to disease progression, death or incomplete response and use of further anticancer therapies) by 4.9% compared with the standard ABVD regimen (82.1% vs 77.2%).3 Accordingly, the relative risk of disease progression with the brentuximab vedotin regimen was significantly reduced by 23% compared with the comparator arm (HR: 0.77 [95% CI: 0.60 –0.98]; p=0.03).3
SEQUENTIAL TREATMENT WITH BRENTUXIMAB VEDOTIN IN ELDERLY PATIENTS
A phase II multicenter study included 48 treatment-naïve cHL patients of over 60 years of age (median: 69 years), who received 2 initial cycles of brentuximab vedotin followed by 6 cycles of AVD.25 Responders continued the treatment with an additional 4 consolidation cycles of brentuximab vedotin. In total, 77% of patients were able to complete 6 cycles of AVD and 73% received at least one consolidation cycle with brentuximab vedotin. This resulted in an overall response rate (ORR) of 82% and a complete remission (CR) rate of 36%. After initial treatment with brentuximab vedotin. After 6 cycles of AVD, the ORR and CR rate increased to 95% and 90%, respectively. The 2-year disease-free, PFS and overall survival (OS) rates were 80%, 84% and 93%. Grade 3−4 adverse reactions occurred in 42% of patients, most frequently neutropenia, febrile neutropenia, pneumonia and diarrhoea. Overall, this novel regimen was well-tolerated and led to encouraging response and survival rates.
CONCLUSIONS
-
Brentuximab vedotin represents an effective and well-tolerated therapeutic option for consolidation therapy in cHL patients after ASCT, even in the presence of adverse prognostic factors.1
-
Promising results were also achieved in the first-line combination therapy of brentuximab vedotin with AVD as well as in the sequential application of brentuximab vedotin-AVD-brentuximab vedotin in elderly patients.25
Informed Consent
General written consent was obtained from the patient for the publication of this case report and any accompanying images.
COI
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The author crafted and approved the final manuscript.
__a_ct_scan_of_the_chest_and_abdomen_rev.png)


__a_ct_scan_of_the_chest_and_abdomen_rev.png)