INTRODUCTION
The evolution in therapeutic strategies in cancer medicine throughout recent decades is tangible, especially in hematological neoplasias. Multiple myeloma (MM), accounting for about 10% of hematological neoplasias, is one of the entities with the greatest progress regarding the mode of actions of newly developed drugs and subsequent benefit for patients. In this review, a summary of concepts and developments in the treatment of multiple myeloma is given, with special attention to the mode of action and precision medicine.
EPIDEMIOLOGY
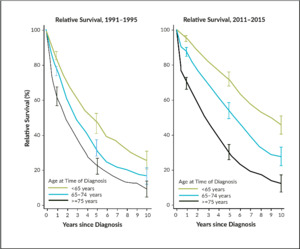
Multiple myeloma is a heterogeneous disease related to an uncontrolled expansion of monoclonal plasma cells and symptoms present through suppressing normal myelopoiesis in bone marrow, bone destruction, kidney damage, and infections. The median age of illness is about 70–75 years, with an incidence of about 4–6 per 100,000. In Switzerland, about 570 patients are newly diagnosed with MM every year, and 440 deaths occur due to the disease. A retrospective analysis of the National Institute for Cancer Epidemiology and Registration (NICER) database demonstrates the impressive progress in 5-year survival rates in Switzerland; increasing from 46.4% to 71.4% (patients <65 years at the age of diagnosis), 31.7% to 53.2% (patients 65–75 years) and from 21.9% to 29.4% (patients >75 years), from the periods between 1991–1995 and 2011–2015.1 These improvements are predominantly attributed to the increasing use of high-dose chemotherapy (HDCT) with autologous stem cell transplantation (ASCT) in younger patients, as well as new anti-neoplastic and supportive drugs. Since the therapeutic armamentarium has increased since 2015, it could be supposed that numbers would be even better nowadays (Figure 1). Nevertheless, the lack of a plateau demonstrates that we are still far from a cure for the disease.
RISK STRATIFICATION
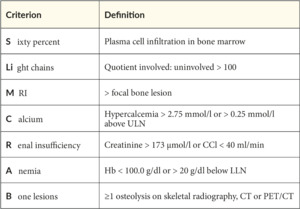
Diagnosis of multiple myeloma is not by itself a trigger of starting therapy immediately. Historically, the CRAB-criteria (Hypercalcemia, Renal insufficiency, Anemia, Bone lesions) were acknowledged to define the transition from smoldering myeloma (which bridges the gap between a monoclonal gammopathy of undetermined significance [MGUS] and MM) to symptomatic MM (SMM). This changed in the early 2010s when the Spanish PETHEMA-GEM group demonstrated the benefit of treating asymptomatic high-risk myeloma early.2 As the definition of high-risk SMM varied widely, the International Myeloma Working Group (IMWG) introduced extended criteria of myeloma-defining symptoms in 2014, the so-called SLiM-CRAB-criteria, which introduced, among others, the number of free light chains and specified the formerly known CRAB-criteria (Table 1).3 The SLiM-CRAB criteria are recognized as the standard for symptomatic myeloma-defining measures, even if other, broader criteria are discussed.4,5 Once the diagnosis of treatment-demanding MM is made, staging according to the revised ISS and risk classification are mandatory (Table 2).6 Further high-risk cytogenetic aberrations allow for patient risk stratification, can provide insight into expected treatment outcomes and remission status, and are reported according to IMWG rules.7
Cytogenetic risk factors
An increasing median overall survival (OS) in MM patients can be largely attributed to an improved understanding of the heterogeneous disease biology and an improved treatment armamentarium. The identification of certain cytogenetic alterations that influence drug resistance and eventual relapse determines the treatment journey.8 However, treatment tends to be a complex, multiple compartment approach comprised of combination and maintenance therapies that is generally dictated by the patient’s condition, utilizing tools like the frailty profile.9
The t(4;14) translocation occurs in approximately 15% of patients with MM and is associated with therapeutic failure and relapse due to overexpression of FGFR3 and MMSET oncogenes.10,11 Overexpression of these oncogenes contributes to tumor establishment and intensifies cell proliferation. Moreover, the less frequent t(4;16) and t(4;20) translocations are associated with upregulation of the MAF and MAFB oncogenes, respectively, and are associated with poor prognosis.12 Furthermore, MAF overexpression is associated with a poor response to the proteasome inhibitors (PIs), bortezomib and carfilzomib.13 Conversely, the t(11;14) translocations, which upregulates CCND1, have shown an improved response to bortezomib therapy.14 Although some contradictory data associates CCND1 upregulation with poor prognosis, disease progression, and increased multidrug resistance 1 (MDR1) gene expression and chemoresistance.15
The deletion of the short arm on chromosome 17 is associated with a dismal prognosis and is associated with advanced disease (due to the loss of the p53 tumor suppressor gene and dysregulation of the cell cycle) and drug resistance. Patients with del17p have shown poor responses to ASCT, lenalidomide, bortezomib, and thalidomide-based therapies.16 Furthermore, the deletion of chromosome 13(q14q21) is apparent in 43% of MM patients, is co-occurring with t(4;14) and t(4;16) translocations in 85% and 92% of patients, respectively, and is associated with a moderate prognosis.17 Despite this, patients with the 13q deletion have shown a similar response to bortezomib as patients without this deletion.18
The deletion of the 1p21 region and the amplification of 1q21 (CKS1B) have also been associated with shorter survival in MM patients.16 The FAM46C and CDKN2C genes are implicated in 1p deletion and are associated with reduced remission and OS in patients who undergo ASCT.19 Furthermore, 1q gains also affect patients who underwent ASCT and confer bortezomib resistance. Nevertheless, immunomodulatory drugs (IMiDs) do not appear to be affected by chromosome 1 abnormalities.20
Various mutations in the drug targets, including proteasome conformation and cereblon activity, also contribute to different degrees of resistance to PIs and IMiDs, respectively. The proteasome subunit beta 5 (PSMB5) is responsible for chymotrypsin-like proteasome activity, is the main target for bortezomib and carfilzomib, and is often mutated in MM patients, leading to resistance.21 Moreover, high expression of cereblon is associated with excellent response to thalidomide, lenalidomide, and pomalidomide, although cereblon mutations causing downregulation are associated with resistance to IMiDs.21,22 Furthermore, MYC oncogene overexpression at the 8q24 chromosome, common in later stages of MM pathogenesis, is associated with disease aggressiveness and resistance to melphalan and bortezomib.23
As evidenced through a range of cytogenetic, molecular, and proliferative risk factors, MM is increasingly recognized as a heterogeneous disease. The Mayo Stratification for Myeloma and Risk-Adapted Therapy (mSMART) consensus opinion tool accounts for genetically determined risk status and the plethora of available treatment options.24 Broadly, the mSMART system classifies patients into 3 risk categories determined by cytogenetic aberrations (Table 3).25 The mSMART system also includes patients who have relapsed <12 months from transplant or progression within first year of diagnosis in the high-risk category.
EVOLUTION OF THERAPEUTIC CONCEPTS OVER THE YEARS
The successful treatment of MM has made tremendous progress in recent decades (Figure 2). The first agent with proven benefit was melphalan in the early 1960s, reaching a median survival of about 3–4 years.26,27 For about 30 years, no other treatment showed an improvement in survival rates compared to melphalan. In the late 1970s, melphalan was escalated to higher dosages, thus paving the way to the concept of HDCT followed by ASCT.28 Starting in the 1990s, this concept is still the preferred choice for younger patients in good health conditions. Whereas formerly reserved for patients <65 years, this concept is now in experienced hands, available for fit patients up to 70 years, or even older in exceptional cases. Various studies (such as IFM-200929 or EMN02/H09530) underlined the ongoing benefit of this concept even against newer drugs and even as a salvage strategy.31 Melphalan remains the standard as a conditional regimen but is challenged by combination with busulfan or bendamustine.32,33 According to a large meta-analysis, many centers provide upfront tandem transplantation for high-risk patients.34 In contrast to purely autologous approaches, auto-/allogenic or allogenic regimens are not as common in MM and should be reserved for fit young patients with high-risk features upfront or after an early first relapse (assumed at least a very good partial response [VGPR] after re-induction and presence of a matched donor).35
DRUGS BEYOND MELPHALAN AND STEM CELL TRANSPLANTATION
Proteasome inhibitors (PI)
Proteasomes are responsible for the degradation of intracellular proteins and, as such, contribute to the maintenance of protein homeostasis and clearance of misfolded and cytotoxic proteins. Proteins targeted for proteasomal degradation become ubiquitinated. After degradation, mediators of apoptosis are released. It is well-known that malignant cells are more susceptive to proteasome inhibition than other cells.37 Bortezomib (Velcade®), a first-in-class proteasome inhibitor (PI), was approved as a second-line treatment in Switzerland in 2005 (approved by the FDA in 2003). Bortezomib directly inhibits proliferation and induces apoptosis of myeloma cells by disruption of intracellular protein metabolism leading to inhibition of cytokine secretion, suppression of adhesion molecule expression, and inhibition of angiogenesis – the latter playing an important role in both MM pathogenesis and disease progression (Figure 3).38 Simply said, the myeloma cell is choked by its own waste. Moreover, carfilzomib (Kyprolis®), an even more potent second-generation PI, became available (approved in Switzerland in 2017). Ixazomib (Ninlaro®) was the first oral PI to be developed and is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with relapsed and refractory MM.
Immunomodulatory drugs (IMiDs)
Thalidomide was introduced into the field of hematological neoplasias at the end of the last century, revealing a promising efficacy in otherwise heavily pretreated myeloma patients.39 Even without official approval, it was widely used by the hematology community until the availability of lenalidomide (Revlimid®), a functional and structural analog of thalidomide, which was approved initially as second-line treatment in 2007. Meanwhile, another thalidomide-derived, third-generation IMiD, pomalidomide (Imnovid®), entered the scene. Whilst the mechanism of action of IMiDs is not completely understood, evidence describes immunomodulatory effects (T-cell co-stimulation, suppression of regulatory T-cells [Tregs], increased production of Th1 cytokines, activation of natural killer [NK] and NK-T-cells), interference with the tumor microenvironment (anti-angiogenesis, anti-inflammation, anti-osteoclastic, modulation of cytokine production, downregulation of adhesion-molecules) and direct anti-tumor effects (cell cycle arrest, induction of apoptosis) (Figure 4).40 In addition, selective inhibition of transcription factors, such as Ikaros, amongst others, is increasingly recognized as an important mechanism.41
Monoclonal antibodies
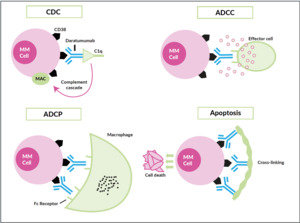
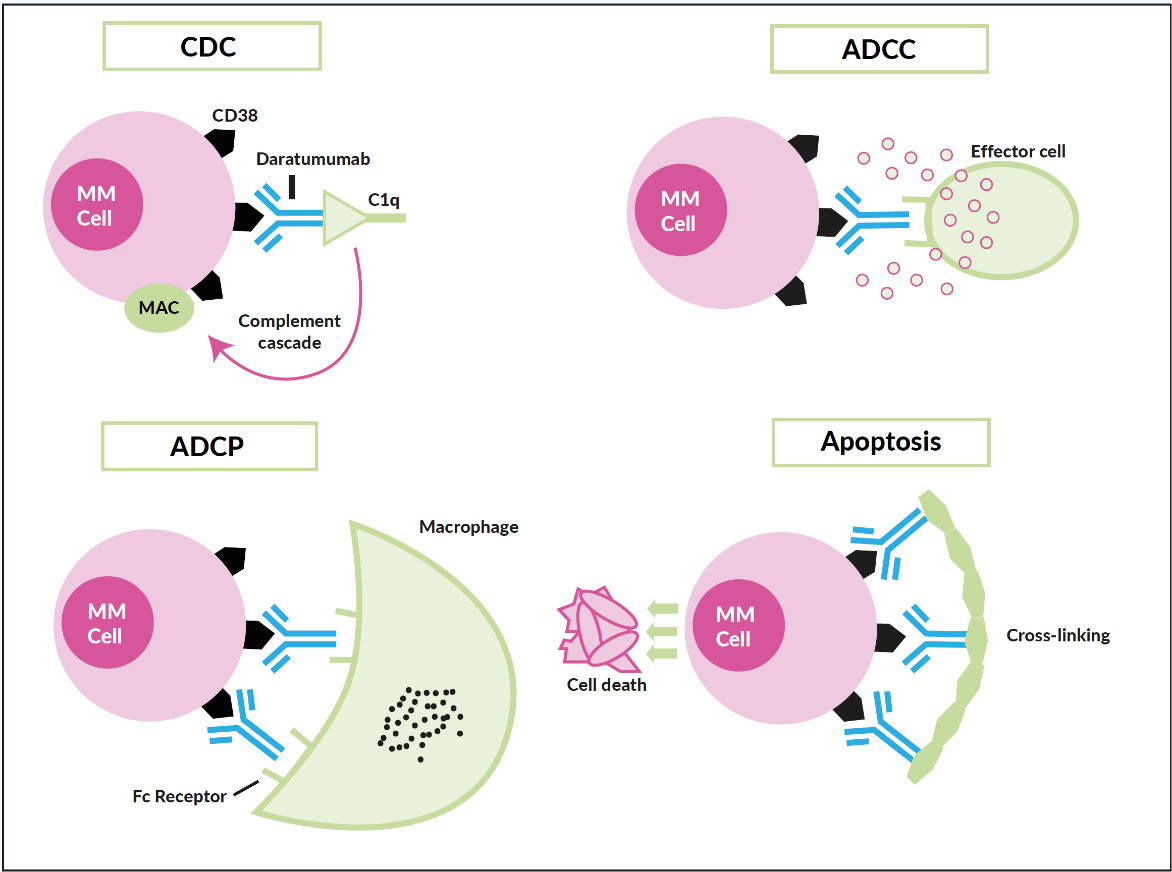
Only a few years ago, humanized monoclonal antibodies, a cornerstone in the treatment of B-cell non-Hodgkin lymphoma, showed up in the myeloma field, yet with two different targets. Elotuzumab (Empliciti®) tags the myeloma cell via binding to SLAM-F7 (also known as CS-1 or CD319) for recognition and degradation by antigen-dependent cellular cytotoxicity (ADCC), and direct activation of NK-cells.42 SLAM-F7 is not uniquely expressed on normal and malignant plasma cells, but also on other cells of the hematopoietic system, including dendritic cells, B- and T-cells, and monocytes. Nevertheless, the overexpression on myeloma cells makes SLAM-F7 a target for immunotherapy. Elotuzumab has proven efficacy in a combination therapy regimen (Table 3) but failed to demonstrate single-agent activity. Daratumumab (Darzalex®) is the prototype of an antibody directed against CD38, another ongoing success story. It was approved in Switzerland in early 2017 as monotherapy after at least 3 prior therapy lines and refractoriness to IMiDs, as well as proteasome inhibitors. Due to a vast and elaborate research program that successfully used different combinations also early in the treatment course, further approvals will be expected soon. Daratumumab binds with its Fc-fragment to C1q (initiating complement cascade resulting in cell lysis), to FcR-bearing effector cells such as NK-cells (leading to activation of cytotoxic processes) and macrophages (inducing phagocytosis), moreover, FcR-mediated cross-linking of daratumumab induces direct cellular apoptosis (Figure 5).43 Like SLAM-F7, CD38 is also expressed by other normal cell subsets, where red blood cells are the most clinically relevant, as difficulties in blood group phenotyping (indirect antiglobulin testing [IAT]) may occur. For several months, isatuximab (Sarclisa®), a second CD38-directed antibody is available in Switzerland.
Histone deacetylase (HDAC) inhibitors
HDAC-6 inhibitors (HDACis) represent a new class of anticancer drugs that target class I and II histone deacetylase enzymes involved in epigenetic regulation of gene expression, which have also been evaluated for the treatment of patients with MM.44 Among these, panobinostat (Farydak®), a hydroxamic acid, is a non-selective pan-HDAC inhibitor, which showed promising results in combination with bortezomib and dexamethasone in clinical studies in MM patients.45 In the phase III PANORAMA1 trial, panobinostat plus bortezomib and dexamethasone were associated with a clinically relevant and significantly prolonged progression-free survival (PFS) in patients with relapsed or relapsed and refractory MM who have received 1−3 previous treatment regimens, as compared with placebo plus bortezomib and dexamethasone. A significantly improved OS in this patient population was not shown.46 Panobinostat was recently approved by Swissmedic.
Supportive treatments
Bone lesions are very common throughout the multiple myeloma disease course. The approval of bisphosphonate zoledronic acid in Switzerland in 2001 marked significant progress by prolonging the time to relevant skeletal-related events and reducing their occurrence. Twenty years later and according to MRC Myeloma IX-Study,47 zoledronic acid is still the most widespread agent in this intention in Switzerland, even if the rank-ligand inhibitor denosumab (Xgeva®) demonstrated non-inferiority and its application seems more convenient.48 The optimal application interval (particularly one versus three months) is the subject of different studies. Adequate calcium substitution is mandatory with both treatment modalities (except present hypercalcemia), as severe hypocalcemia were reported as well-known side-effects.49
Steroids are a supportive treatment as anti-neoplastic agents (through directly mediated apoptotic effects) as well. Common is the use of prednisolone and dexamethasone, mostly as pulse therapeutic regimen with different combination partners (Table 3), but also moderate single-agent activity. The latter makes dexamethasone an attractive agent for the immediate start of therapy when multiple myeloma is suspected but diagnostic procedures are not yet completed.50
Antimicrobial prophylaxis with TMP/SMZ and virostatics are widely used during MM therapy with respect to common myeloma- and therapy-dependent immunodeficiency and steroid use. Virostatics as valaciclovir are mandatory as co-medication to proteasome inhibitors and recommended by use of monoclonal antibodies. Equally important are vaccinations against pneumococci and yearly against influenza. As the incidence of varicella-zoster virus (VZV)-reactivation in myeloma patients is quite high, the rates of vaccinations against VZV are increasing, preferring non-live recombinant glycoprotein E51 over the live-attenuated vaccine.52 Symptomatic hypogammaglobulinemia should lead to a monthly infusion of intravenous immunoglobulins. The use of TMP/SMZ is recommended, especially during the first 2–4 months of myeloma-therapy.53
As with other types of cancer, myeloma patients are at increased risk for thromboembolic events, especially for patients under treatment with IMiDs. They should receive at least a prophylaxis with acetylsalicylic acid (if no further risk factors are present) or low molecular weight heparin (Figure 6).
Lastly, radiotherapy is a widely used and successful instrument for stabilizing bone lesions, sparing pain killers such as narcotics, and reducing the otherwise refractory extramedullary spreading of disease.55
PROMISING THERAPEUTIC CONCEPTS ANTE PORTAS
Some very promising novel therapeutic modalities will enter the field of anti-myeloma treatment in the upcoming years:
-
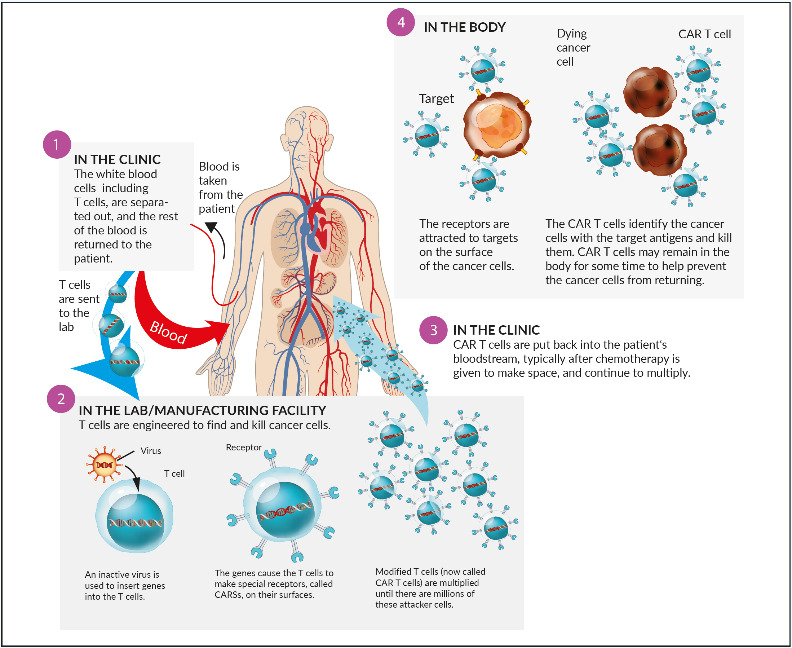
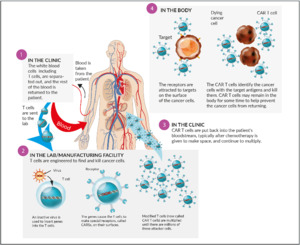
Chimeric antigen receptor T- (CAR T) cells: The concept of transfected, engineered T-cells with a modified chimeric receptor directed against tumor cells (Figure 7) was successfully introduced in the treatment of acute lymphoblastic leukemia (ALL) and advanced aggressive B-cell-lymphomas. Targeted against CD19, both tisagenlecleucel (Kymriah®) and axicabtagene ciloleucel (Yescarta®) became available in Switzerland recently. Currently, no CAR T-cell product against myeloma is available in Switzerland. The most advanced target in myeloma cells seems to be the B-cell-maturation antigen (BCMA), which is expressed on myeloma cells, but virtually absent on naïve and memory B-cells. A phase I study revealed an objective response rate (ORR) of 85% and a complete response rate (CR) rate of 45% in heavily pretreated patients. Currently, unlike CD19-directed CAR T-cells in B-cell-lymphoma, long-term durable remissions appear to be more scarce.56
-
Bispecific T-cell engagers (BiTEs): like the CAR T-cells, BiTEs have already been successfully introduced in relapsed/refractory ALL (blinatumomab) treatment. In multiple myeloma, AMG420 is an investigational BiTE-molecule binding to BCMA on target cells, resulting in T-cell-mediated lysis of myeloma cells. A phase I study demonstrated an ORR of 70% in patients with ≥2 prior therapies.57 Furthermore, an early-phase trial of CC-93269, a BiTE that binds to BCMA on malignant plasma cells and CD3 on T-cells, has shown a promising ORR (83.3%) and a manageable safety profile in a heavily pretreated patient population.58,59
-
Immunoconjugates: this therapeutic class is well-known for Hodgkin lymphoma and CD30-positive T-cell-lymphoma (brentuximab vedotin/Adcetris®), and also increasingly from solid tumors. Immunoconjugates are antibodies directed against a preferably specific antigen on tumor cells, conjugated by a protease-resistant linker to a microtubule-disrupting agent (often monomethyl auristatin F [MMAF]). Belantamab mafodotin is a first-in-class anti-BCMA immunoconjugate that kills myeloma cells via a multimodal mechanism, including delivery of MMAF to BCMA-expressing multiple myeloma cells (thereby inducing apoptosis), enhancing ADCC and ADCP (phagocytosis) and inducing immunogenic cell death. A phase II study demonstrated an ORR of about one third in a really heavily pretreated population (including refractoriness to IMiDs, PIs, and anti-CD38 antibodies).60
-
Selinexor is a selective inhibitor of Exportin 1 (XPO1), which as the sole known nuclear exporter of tumor suppressor proteins, is overexpressed in myeloma, forcing among others the nuclear localization and functional activation of tumor-suppressor proteins. The result is the induction of apoptosis. In a phase II study on a heavily pretreated population, about one quarter received at least a partial response to selinexor combined with dexamethasone.61
-
Venetoclax is a well-known B-cell lymphoma 2 (BCL2) inhibitor with exceptional efficacy in chronic lymphocytic leukemia (CLL) and other low-grade lymphomas and is increasingly used in high-grade myelodysplastic syndromes (MDS) and AML. Early studies in multiple myeloma revealed promising single-agent activity, especially in heavily pretreated patients harboring the t(11;14)-translocation.62 The recently published phase III BELLINI study confirmed the high efficacy of the combination venetoclax/bortezomib/dexamethasone against bortezomib/dexamethasone regarding PFS (OS not yet mature in this population) in myeloma with high BCL2-expression and/or t(11;14). On the other side, serious safety concerns compromising OS were raised in patients without the mentioned characteristics. This leads to the suggestion that a biomarker-driven approach might be appropriate for the use of venetoclax in the treatment of multiple myeloma.63
TREATMENT ALGORITHMS IN 2020
Treatment of MM has become more and more challenging over the last two decades, for many different reasons. Firstly, as more and more substance classes became available and different drugs in multiple combination regimens have been explored, it is critical to bear in mind an adequate therapeutic strategy, including following therapeutic lines, from the outset. Second, the merry-go-round turns fast, meaning that it is quite difficult to keep an overview of the different substances and combinations. Third, not all drugs and possible combinations are approved and reimbursed in a timely manner by regulatory instances. Fourth, supportive therapy and possible dose modification requests require a lot of experience, even if recommendations exist.65 Fifth, the importance of deep and sustained remissions has become increasingly clear, leading to often continuous and demanding therapies over the years without interruption, stressing the adherence of patients.
As hematologists/oncologists involved in the treatment of MM, we should choose the best treatment strategy for our individual patient considering age, fitness (performance score), co-morbidities (making use of assessment tools like R-MCI or HCT-CI), expectable side effects, therapeutic goals, domicile, drug-availability, and other factors.66,67 Reimbursement should, in any case, be assured before starting a therapy not yet Swissmedic-approved (particularly combinations) or not yet on the SL (“Spezialitätenliste”) of the Federal Office of Public Health (FOPH).
Actually, for most fit and young patients up to 70 years of age, 3–4 cycles of a triplet-induction therapy (usually lenalidomide plus bortezomib plus dexamethasone [VRd]) followed by mobilization chemotherapy (in Switzerland usually navelbine), stem cell collection, HDCT (usually melphalan) and ASCT is suggested.68 It is well known that frontline ASCT is associated with a higher rate of minimal residual disease (MRD)-negativity and that an MRD-negative status has a favorable prognostic impact. As response rates, depth of response and PFS are surrogate markers for the outcome, most potent therapies should be given best first.69 High-risk patients usually get a second (tandem) HDCT/ASCT, the value of consolidation therapy for the other patients is not clear. All patients should nowadays receive maintenance therapy (usually lenalidomide, except high-risk patients, who receive bortezomib rather, if reimbursed by health insurance) for at least 2 years, ideally until progression. At the time of relapse, most fit and young patients should undergo a second HDCT/ASCT (if not already transplanted into a tandem-concept and if the response to the first HDCT/ASCT was sustained). All others should switch to a non-transplant scheme (Table 4), incorporating composition and response to already obtained therapy.
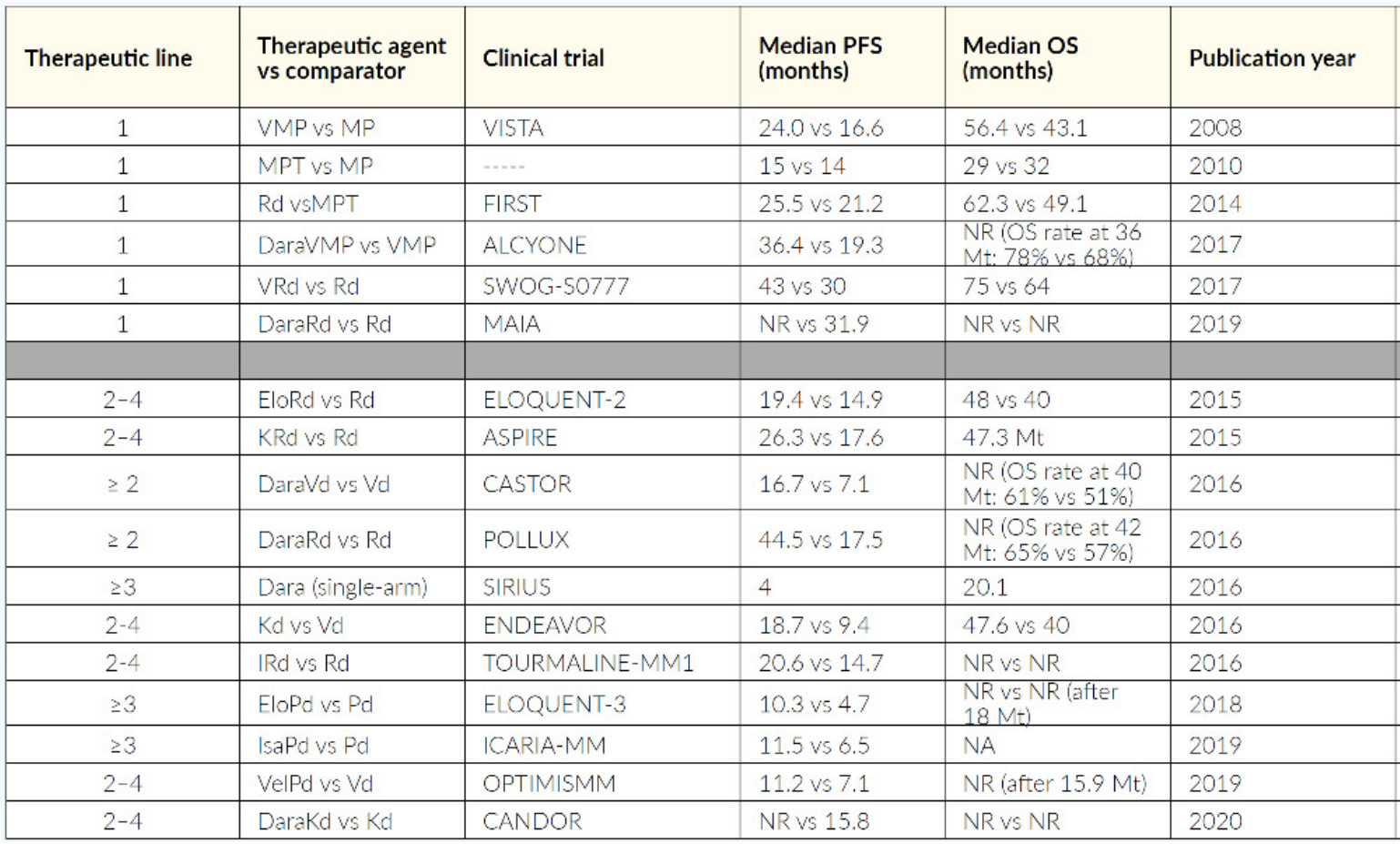
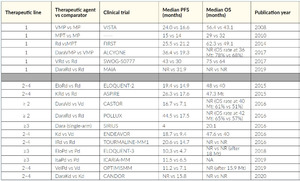
Patients not qualifying for HDCT are usually treated with a triple or quadruple first-line therapy like daratumumab plus lenalidomide and dexamethasone (Dara-Rd), Dara-bortezomib/melphalan/prednisone (Dara-VMP), VRd (lite), VMP. Only frail patients receive a doublet like lenalidomide plus dexamethasone (RevDex) or bortezomib plus dexamethasone (VelDex). Anti-CD38 antibodies should be used as early as possible and as part of a combination regimen. If a patient at the beginning is not clearly transplant-eligible or -ineligible, VRd is currently the preferred first-line option. It is important that a patient has the opportunity to undergo as many different classes of therapy (like proteasome inhibitors, IMiDs, monoclonal antibodies) as possible, refractoriness to one class should not be assumed, as long as third-generation IMiDs and second-generation PIs are not already used. An excellent overview of therapeutic strategy is detailed by recommendation of Swiss Experts (Figure 8), and a selection of relevant studies is outlined in Table 3.70
CONCLUSION
Progress made in taking care of our multiple myeloma patients over the last decades is impressive. Improved understanding of complex biological, pathophysiological, and molecular mechanisms has led to the development of drugs with different therapeutic targets. Furthermore, deepened and sustained remissions translate into conspicuously longer survival of the patients, who now tend to survive 8–10 years, with some individuals reaching 15 years or even more. Nevertheless, survival curves deny a plateau, and no cure is in sight. Our goal must be to further improve outcomes by supporting myeloma research, enroling patients in studies where possible, making innovations accessible to individual patients in a timely manner, and guiding our patients through often long-lasting and time-consuming therapies, weighing the individual therapeutic measures and quality of life carefully.
TAKE-HOME-MESSAGES
-
Multiple myeloma (MM), also known as plasma cell myeloma, is a malignancy of the plasma cells. It is characterized by neoplastic proliferation of plasma cells and accounts for 10% of all blood cancers.
-
The therapeutic landscape of MM has advanced significantly over the past decades. Due to the heterogeneous nature of the disease, treatment decisions are based on a personalized approach. This involves risk stratification, assessment of patient co-morbidities, and consideration of treatment history and response.
-
Several novel treatment regimens (quadruplet, triplet, and doublet regimens) are currently being used for the treatment of MM, and new combinations are constantly being developed.
-
Future treatment options include chimeric antigen receptor T- (CAR T) cells, bispecific T-cell engagers (BiTEs), and immunoconjugates. Several new compounds (like belantamab mafodotin, melflufen, venetoclax, and selinexor) are in the pipeline and are currently being tested in phase III trials in newly diagnosed and relapsed/refractory MM patients.
CONFLICT OF INTEREST
AS received consultancy honoraria and travel grants from Novartis, Takeda, Incyte, Sanofi, Celgene, Amgen, and Janssen. The author declares that these relationships had no influence on the content of the article.
Author Contributions
The author crafted and approved the final manuscript.
_and_risk_categories_for_multiple_myeloma.__656.png)



_drugs.png)
__risk_factors_and_recommended_prophylaxis.png)



_and_risk_categories_for_multiple_myeloma.__656.png)



_drugs.png)
__risk_factors_and_recommended_prophylaxis.png)