INTRODUCTION
Worldwide, breast cancer is the most common cancer in females, with an annual incidence rate of 80–113 cases per 100,000 women.1 The incidence has increased through recent decades, but the mortality rate has steadily dropped over the last 30 years.2 Rising incidence is attributed to a combination of better diagnostic methods, lifestyle changes, hormonal replacement therapies, improved screening initiatives, and earlier detection.2 The declining mortality rate is also associated with screening, together with modernized treatment options, including surgery, chemotherapy, targeted therapy, and radiotherapy. Today, 85% of patients with a breast cancer diagnosis can be cured with multimodal therapy.3 However, while the aim of treatment is to cure, quality of life and prevention of acute and late treatment-associated toxicity is paramount to the decision process.4
The St. Gallen Consensus defines four clinically relevant subtypes of breast cancer: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and triple-negative disease (TNBC) subtypes (Table 1). This basic classification is predominantly based on immunochemistry (IHC) markers, including estrogen receptor (ER), progesterone receptor (PR), ERBB2/HER2, and Ki-67. These clinically established, yet simple targets generally correlate with the intrinsic subtypes and still help to guide treatment decisions today. However, through whole-genome sequencing, single-cell analysis, and proteomics, new targets are on the horizon to improve treatment decision-making and outcomes.5
In the era of precision medicine, it is generally accepted that breast cancer is characterized beyond ER/PR/HER2; each subtype requires specific treatment regimens, thus necessitating the evolution of the breast cancer treatment landscape. Recent data from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) Study demonstrated 10 molecular subtypes with distinct, yet comparable, clinical outcomes.7 This group performed an integrated analysis of copy number and gene expression in a discovery group of 997 primary breast tumors, and a subsequent validation set of 995 samples, to discern reproducibility, with long-term clinical follow-up.
In general, for HER2-positive and TNBC disease, neoadjuvant systemic therapy is applied before surgery.8 The pathological complete response (pCR) rate in TNBC patients has increased through the use of immune checkpoint inhibitors plus standard chemotherapy.9 In HER2-positive disease, dual inhibition of the HER2 kinase with trastuzumab and pertuzumab plus chemotherapy increased pCR rate and event-free survival.10,11 Adjuvant trastuzumab emtansine is another new strategy for patients with HER2-positive disease who do not achieve pCR.12
In luminal A/B disease, about 25% of patients receive chemotherapy after primary surgery. Genetic signatures like the Oncotype DX®, MammaPrint®, EndoPredict®, Predictor of Microarray 50 (PAM50), and others help to identify tumors that can potentially benefit from additional chemotherapy.13–16 Today, chemotherapy is still based on anthracycline and taxanes regimens.8 A recent randomized study, which included over 10,000 women with hormone receptor-positive/ HER2-negative breast cancer, demonstrated the beneficial role of chemotherapy in patients with intermediate or high-risk disease, with certain risk factors, including early age, menopausal status and midrange 21-gene recurrence score.13
By shortening the intervals between therapy cycles or delivering individual drugs at full dose sequentially, the intensity of cytotoxic therapy can be increased with relative safety. This dose-dense concept is becoming increasingly important, especially in patients with high-risk tumors, e.g., high grading, increased lymph node involvement, and high proliferation.17–20 These drug delivery methods have shown improved relapse-free survival. A recent meta-analysis from the Oxford-Overview group clearly emphasizes the role of a dose-dense regimen for improved clinical outcomes.18 On the other hand, the potential long-term toxicities, such as hematologic malignancy, heart and lung disease, as well as clinical benefits, should be considered in patient’s informed consent.21–23
TUMOR HETEROGENEITY IN THE ERA OF PRECISION MEDICINE
Relapse will occur in around 20% of breast cancer patients.1 The most relevant relapse risk factors are grading, disease stage, and breast cancer subtype, including TNBC and HER2- positive disease.24,25 A recently published overview of luminal breast cancer, with a very long follow-up of 20 years after treatment cessation, showed a relapse rate of more than 40% in patients with a high number of lymph nodes over a follow-up time of 25 years after initial diagnosis.26 Tumor heterogeneity might be the most important reason for early and later relapse in all breast cancer subtypes.27 In addition to heterogeneous ER, PR, and HER2 expression among patients with breast cancer, different expression levels between primary cancer and matched metastasis has been observed.28 Nowadays, new techniques like gene-expression profiling or massively parallel sequencing have helped to identify the genetic background of primary and metastatic breast cancers.29,30
Based on a recently published meta-analysis, there is a wide range of expression levels for ER, PR, and HER2 in primary and metastatic breast cancer.31 In this analysis, more the 4,000 tumors were examined. Pooled proportions of tumors shifting from positive to negative and from negative to positive were 24% and 14% for ER (p=0.0183), 46% and 15% for PR (p<0.0001), and 13% and 5% for HER2 (p=0.0004). The change of expression profile has a major implication on treatment selection, and biopsy of the metastatic site is strongly recommended in patients with relapsed metastatic breast cancer.
Based on current research, there are different types of tumor-associated heterogeneity, including intertumoral and intratumoral heterogeneity.7 The management of intertumoral heterogeneity patients and tumors should include patient stratification based on molecular profiling. In addition, treatment trials need to have innovative designs, including master protocols, basket trials, and adaptive trial design. For a better characterization of intratumoral heterogeneity, a metastatic biopsy is necessary to determine the profile of the tumor. Furthermore, a longitudinal tumor follow-up, including repeated metastatic biopsy is necessary. Finally, techniques like NGS, bioinformatics tools and animal models (PDX models) should be used in order to identify driver- events and mutations.28
MOLECULAR SUBTYPES OF BREAST CANCER AND PRECISION MEDICINE (ADVANCED BREAST CANCER)
Immune checkpoint blockade and combinations in TNBC
Triple-negative breast cancer (TNBC), a heterogeneous breast cancer subgroup, is defined by the absence of detectable ER, HER, and PR expression.32–34 In 2011, Lehmann et al. defined 6 subgroups of TNBC with different outcomes and therapeutic implications (Table 2).34 TNBC is associated with poor clinical course, younger age, and hereditary cancers when compared with HER2-positive and ER-positive breast cancers. There is also an association of TNBC and BRCA1/2 mutations and homologous recombination deficiency (HRD).35 Patients with TNBC frequently have a poor prognosis with a relapse rate of 30% in the primary setting. In the last decades, fewer new drugs have been approved in the metastatic setting.
Considering the molecular landscape of TNBC, more than 90% of tumors have molecular alterations in at least one signaling pathway. These include PIK3CA/AKT/mTOR (~40%), cell-cycle pathways such as RB1, CDK2, 4, 6, and others (40%) and RAS/MAP-kinase pathway (~12%). In addition, overexpression of growth factor receptors (GFR) including EGFR, MET, and EGF1R (~15 %) and deficiencies in DNA repair including BRCA (~12%) has been reported in TNBC tumors.36
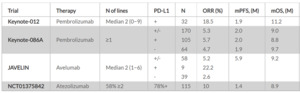
Besides targeting molecular alteration, immunotherapy has emerged as another important treatment strategy.37,38 So far, the current treatment option with a single-agent immune checkpoint inhibitor showed modest treatment responses. The KEYNOTE-012 study was a phase Ib study that demonstrated an objective response rate (ORR) of 18.5% in heavily pretreated TNBC who were programmed death-ligand 1 (PD-L1)-positive (n=32).39 Further research showed an important role of tumor-infiltrating lymphocytes (TIL) and programmed cell death protein 1 (PD1)/PD-L1 status as an important biomarker in the metastatic setting (Table 3).
In 2019, the TONIC trial evaluated several combination strategies with nivolumab, a programmed cell death protein 1 (PD-1) inhibitor.40 In this study, 67 patients underwent randomization to receive either nivolumab as monotherapy or in combination with a) irradiation, b) chemotherapy with doxorubicin, c) low-dose cyclophosphamide, or d) chemotherapy with cisplatin, all followed by nivolumab maintenance therapy. The highest response rates were reported in the doxorubicin (23%) and cisplatin (35%) cohorts. After doxorubicin and cisplatin induction, the upregulation of genes involved in PD-1/PD-L1 and T-cell cytotoxicity pathways was observed. Results further showed upregulation of JAK-STAT and TNF-α signaling in the doxorubicin cohort and combinations with this mechanism might be promising for further evaluation. The trial concluded that the combination of doxorubicin/cisplatin and immunotherapy with nivolumab might induce a more favorable tumor environment and increase the likelihood of response to PD-1 blockade. Further analysis and clinical trials are needed to define the best combination regimes.
IMpassion130, a landmark trial published in the New England Journal of Medicine, showed that a combination of nab-paclitaxel and atezolizumab, a PD-1 inhibitor, provide an overall survival (OS) benefit among TNBC patients with PD-L1-positive tumors (the expression on tumor-infiltrating immune cells ≥1%) compared with single-agent nab-paclitaxel (25.0 months vs 15.5 months; HR: 0.62 [95% CI: 0.45−0.86]).41 These data also indicated that the biomarker- derived approach and the combination of chemotherapy and immunotherapy is an important treatment strategy in this patient population.42,43 As a result, this drug combination has become the standard of care for metastatic PD-L1-positive TNBC. However, atezolizumab plus paclitaxel recently failed to show an improvement in progression-free survival (PFS) compared with paclitaxel alone in PD-L1 positive patients in the IMpassion131 trial. Furthermore, a negative trend for OS was observed, although data are immature and not sufficiently powered.44 Future perspectives include a combination strategy of different immune therapy agents and chemotherapy.
Currently, a large phase III trial is evaluating the combination of chemotherapy, immunotherapy and targeted therapy with ipatasertib, a novel small molecule inhibiting AKT, an important component of the cancer pathway PIK3CA.45 Previously, in preclinical models, ipatasertib demonstrated activity against all three isoforms of AKT.46 Furthermore, in a first-in-human phase I study, ipatasertib monotherapy showed clinically meaningful tumor control (disease control rate of 30% [16/52 patients]) and manageable toxicity in patients with solid tumors (median prior lines of treatment: 6 [1−17]), including breast (31%), colorectal (27%), prostate (12%) chondrosarcoma (4%), ovarian (4%) and other (22%) tumors.47 The most common toxicity was gastrointestinal toxicity of grade 1−2.
Ipatasertib was also assessed in patients with TNBC. The LOTUS trial investigated the addition of ipatasertib to paclitaxel as first-line therapy in patient with TNBC.48 In this phase II trial, 124 patients with advanced metastatic TNBC received paclitaxel (80 mg/m2 on days 1, 8, 15), with or without ipatasertib (400 mg on days 1–21). PTEN status was assessed in all patients. The co-primary endpoints, PFS in the intention-to-treat population, and PFS in the PTEN- low (by IHC) patients, were 6.2 months versus 3.9 months, respectively (HR: 0.60 [95% CI: 0.37–0.98]; p=0.037).
Further evaluation in TNBC was recommended by the authors. Ipatasertib has been further evaluated with a modern anti-PD-L1 inhibitor and chemotherapy (paclitaxel weekly) within the IPATUNITY studies.49 Preliminary results from this phase Ib study demonstrated that a combination of ipatasertib plus atezolizumab and paclitaxel/nab-paclitaxel provided a response rate of 73% in patients with locally advanced/metastatic TNBC, irrespective of biomarker status, with a manageable toxicity profile.
HER2-directed therapy in advanced HER2-positive breast cancer
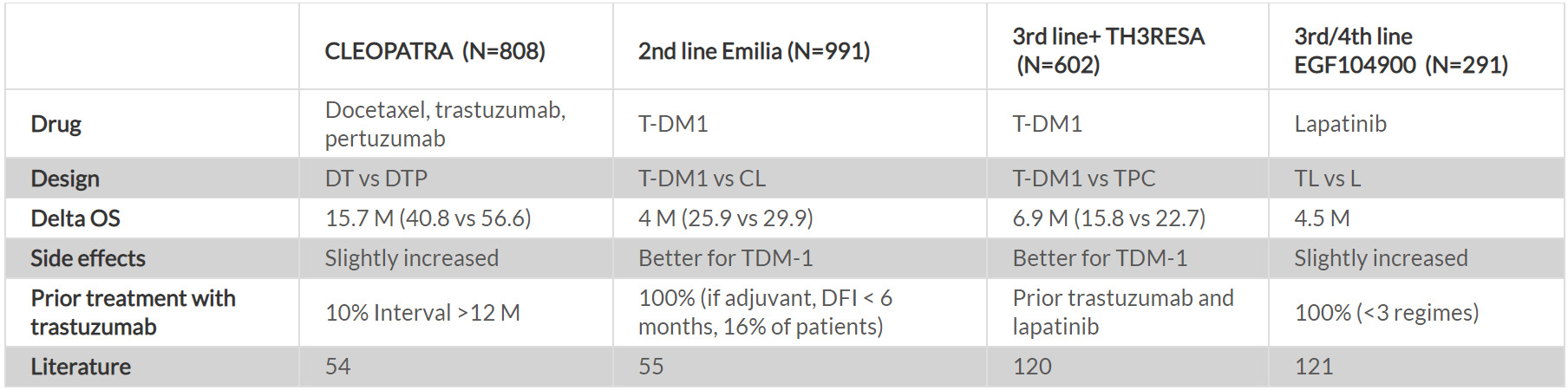
HER2-positive breast cancer accounts for up to 15% of breast cancer and is characterized by the overexpression of the receptor tyrosine kinase HER2.50 In general, the prognosis in this breast cancer subtype is poor, with an aggressive course of the disease.51 However, several new systemic treatment options have improved survival outcomes over the last two decades. For example, trastuzumab has provided a significant clinical benefit in patients with metastatic HER2-positive breast cancer, which led to the approval of this agent for the treatment of this patient population more than 18 years ago.52,53 In addition, trastuzumab, the current standard of care, is associated with improved clinical outcomes in the adjuvant setting.8 Within the last five years, several new agents have been approved for the use in HER2-positive patients with metastatic breast cancer (Table 4; Figures 1−3). Among patients without pCR, who represent a group with the worst clinical outcomes, trastuzumab emtansine (TDM-1) provided a considerable survival benefit. In this subpopulation, TDM-1 was associated with a 3-year disease- free survival of almost 90%.12 In addition to TDM-1, only multimodal therapy, including neoadjuvant chemotherapy with trastuzumab/pertuzumab, optimal surgical approach, radiotherapy and endocrine therapy has resulted in such high survival rates. Therefore, this treatment regimen has led to an optimal treatment with the best available prognosis.8
In the metastatic setting, the common standard of care is the combination of pertuzumab plus trastuzumab and docetaxel, based on the CLEOPATRA study.54 This trial demonstrated a tremendous OS benefit of 16 months with this triplet combination. In the second-line therapy, the antibody-drug conjugate (ADC) TDM-1 is the standard of care.55
Later line options include lapatinib, chemotherapy/ trastuzumab and lapatinib/trastuzumab. Landmark clinical trials investigating these treatment regimens are outlined in Table 4. Recently, other agents including tyrosine kinase inhibitors (TKIs) like neratinib, tucatinib, and pyrotinib, as well as ADCs like trastuzumab deruxtecan showed promising activity.56–58
To further optimize treatment in the metastatic setting, several novel strategies have been evaluated in clinical practice and randomized controlled trials (Figure 2). One important strategy seems to be the development of ADC. Trastuzumab deruxtecan is a potent ADC build of a trastuzumab antibody linked with a potent cytotoxic topoisomerase inhibitor. The ADC has a very high payload of chemotherapy with better diffusion agents.59 The phase II clinical trial (DESTINY- Breast 001) evaluated trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer who had received a median of 6 prior lines of therapy (range: 2−27), including treatment with TDM1.57 In this two-step study, patients received 5.4 mg/kg trastuzumab deruxtecan every 3 weeks intravenously (N=184). The response rate was 60.9%, whilst the disease control rate was 97.3%. After a median follow-up of 11.1 months, the median PFS was 16.4 months. Based on this encouraging result, this therapy received FDA approval. Several clinical trials are on the way to determine the role in different clinical settings.60
HR-positive breast cancer: Endocrine therapy, CDK4/6 inhibitors and beyond
Patients with hormonal receptor-positive (HR+) breast cancer are at increased risk of late relapses after 5 years.61 In fact, in high-risk node-positive HR-positive patients with >3 lymph nodes, the 5-year relapse rate is up to 40%.26 Tamoxifen was the standard of care in metastatic HR+ breast cancer, as it provided a high clinical benefit rate and a PFS of around 12−15 months in the primary setting.62 In premenopausal patients, the addition of gonadotropin-releasing hormone (GnRH) analogs to tamoxifen improved both, response rate and OS in a randomized, prospective clinical trial.63 In addition, treatment with GnRH analogs alone or in combination with tamoxifen leads to recurrence-free survival and OS outcomes comparable with those achieved by different chemotherapy protocols in patients with HR+ breast cancer.62 In this patient population, adjuvant therapy with GnRH agonists and tamoxifen is also associated with the preservation of reproductive function.64,65
Aromatase inhibitors (AI) have been in clinical use for two decades, showing improved clinical outcomes in postmenopausal and premenopausal patients with metastatic breast cancer.66–68 However, a high proportion of patients with metastatic disease develops endocrine resistance leading to disease progression within 12−24 months.69–71 In the first-line endocrine therapy setting, the clinical benefit rate is around 60%. Subsequent lines have a clinical benefit rate of 40%, 24% and 16%.72 Beyond third-line therapy, AIs are not beneficial in most patients and a switch to chemotherapy is recommended.8
Clinically endocrine resistance can be divided into primary and secondary (acquired) type.73 Primary endocrine resistance is defined as a relapse less than 2 years after finishing adjuvant endocrine therapy or progression of disease within 6 months on endocrine therapy in the metastatic setting. Secondary resistance is defined as a relapse less than 12 months after finishing endocrine therapy or progression later than 6 months on endocrine therapy for metastatic disease.
The current research has strongly focused on elucidating mechanisms of resistance, and several mechanisms involved in the development of endocrine resistance have been identified.74 One of them is the loss of steroidal receptors during disease progression. A Swedish cohort study demonstrated discordance rates in biomarkers between the primary tumor and corresponding relapse ranging from 14% for ER and 39% for PR. This study also showed that loss of ER or PR in the relapse was associated with a 3-fold increase of death risk compared with patients who had stable ER- or PR- positive tumors.75 Therefore, patients with recurrent disease should receive a biopsy in order to confirm tissue diagnosis and measure the expression of biomarkers, which subsequently determinate the therapy course.75–79 In the era of precision medicine and targeted management, the availability of fresh tissue has become increasingly important to determine optimal treatment approach.29
A number of approaches have been proposed to overcome the endocrine resistance. One of the strategies is to combine endocrine therapy with targeted agents.80 Here, several pathways and alterations involved in breast cancer have been utilized, including alterations and mutations in the ESR1 gene coding for the estrogen receptors.81–83 So far, several ESR1 mutations have been described.84,85 To overcome the endocrine resistance due to ESR1 mutations, selective estrogen receptor degradation (SERD) drugs have been used for the treatment of patients who failed after AI or tamoxifen therapy.86 Fulvestrant, the commonly known SERD, showed promising activity in the advanced endocrine resistant setting.87,88
Further resistance mechanisms are caused by amplification and upregulation of co-activators, as well as alterations in co-repressors, e.g., AIB1 and MNAR/PELP1.89–91 Promising targets are pathways with a cross-talk to the steroidal hormonal pathway. Most studied alterations involve cyclin-depended kinase 4 and 6 (CDK4/6) proteins such as PIK3CA, ESR1, CCND1, FGFR1, BRCA1, BRCA2, AKT1 and HER2.
Several drug combinations are now under investigation. Targeting the PIK3CA pathway seems to be effective, however, most analyses are still experimental and look at a combination of factors, such as mTOR inhibitors.92–94 So far, pan-PI3K inhibitors, such as buparlisib, pictilisib, and SAR245408, have not shown impressive efficacy, whereas PI3K-α-specific inhibition has shown more promise.
Recently, the alpha-specific PIK3CA inhibitor alpelisib was approved in the metastatic setting in ER+ PIK3CA-mutated metastatic breast cancer.95 Data from the SOLAR-1 study demonstrated a substantial PFS benefit in patients treated with alpelisib plus fulvestrant compared with patients treated with placebo plus fulvestrant (11.0 months [95% CI: 7.5−14.5] vs 5.7 months [95% CI: 3.7−7.4]). The drug is well-tolerated, with the most common side effects being hyperglycemia, gastrointestinal toxicity and skin toxicity.
Everolimus, an mTOR inhibitor, showed good clinical outcomes in the endocrine resistance setting.96 In a large randomized, phase III trial, the combination of everolimus and exemestane led to significantly improved PFS compared with placebo plus exemestane (median PFS, 10.6 months vs 4.1 months; HR: 0.36 [95% CI: 0.27−0.47]; p <0.001) in HR+, HER2- patients with advanced breast cancer with prior exposure to nonsteroidal aromatase inhibitors (NSAIs). Based on the trial results, the drug was approved by the FDA and EMA for patients with metastatic breast cancer that had received previous treatment. Everolimus was also tested in a combination of tamoxifen and fulvestrant, where it showed comparable clinical benefits.97,98 Currently, everolimus is being investigated in the adjuvant setting.
Targeting CDKs 4/6, the key regulators of the cell cycle seems to be even more promising. The expression of the D type cyclin, another important component of the cell cycle, is upregulated by several mitogenic signaling pathways, including steroid hormones (such as the ER pathway), PI3K/AKT/mTOR, MAPKs, WNT/β-catenin, STATs, and NF-κB/IKK.99 The formation of a complex of cyclin D with CDK4/6 leads to the hyperphosphorylation of the retinoblastoma (Rb) protein, which in turn activates the E2F transcription factors and the progression of the cell cycle.100
Currently, three CDK4/6 inhibitors are approved by the FDA: palbociclib, ribociclib, and abemaciclib.101–103 The approvals were based on findings from large phase III studies including MONALEESA (ribociclib), PALOMA (palbociclib), and MONARCH (abemaciclib).104–109 All studies showed a clinical benefit of these three treatment regimen versus the standard of care endocrine therapy, e.g., AI or fulvestrant in both treatment-naïve patients and in those that had relapsed or progressed during prior endocrine therapy. The trials showed significantly improved PFS, with hazard ratios ranging between 0.5 and 0.6. The most common side effects included neutropenia, although neutropenic fever was rare, with only 1−2% of all cases. Table 5 provides an overview of the most frequent adverse events associated with the three treatment regimens. Although these drugs are generally well-tolerated, there are some differences in the toxicity profile. Most importantly, however, the combination of the new therapies promises an estimated time of endocrine treatment of more than 36 months before switching to a chemotherapy regime.
Treatment beyond the current classification based on the molecular profile
Currently, the treatment of early-stage and metastatic breast cancer is based on established, yet simple, molecular markers, including ER, PR, HER2, which have prognostic and predictive value.8 Recently, BRCA1/2 and PIK3CA mutational status as well as PD-L1 expression status have been introduced into clinical practice and demonstrated clear clinical benefit for the treatment with olaparib/talazoparib, alpelisib, and atezolizumab.41,110,111
Recently, the Breast International Group (BIG) has initiated AURORA, an ongoing, multinational molecular screening program for patients with advanced breast cancer, with the aim to generate new insights in the molecular treatment of breast cancer.112,113 In total, around 1,300 patients with metastatic breast cancer (≥1 line of systemic treatment for advanced disease), have been planned to be included. In this trial, archival and fresh tissue samples of metastatic lesions and blood are analyzed by NGS for a comprehensive cancer panel of breast cancer-related genes. In a first pilot study using an Ion Torrent sequencing platform at a central facility, 73% of patients had at least one targetable alteration. Orthogonal validation, which was done by Illumina sequencing technology, resulted in an average of 66% concordance of substitution calls per patient. Similarly, copy number aberrations obtained from the Ion Torrent sequencing were concordant in 59% with single nucleotide polymorphism (SNP) arrays, demonstrating that the next-generation genomic techniques are applicable to international molecular screening programs in routine clinical settings. In addition, the primary analysis of the study presented at the ESMO Breast Cancer 2019 congress included molecular results of 381 patients (accrual by November 2017). Among them, pathological subtype distribution was 232 patients with HR+/HER2-, 69 patients with HER2+, 77 patients with TNBC, and 3 with not available subtype. The study identified the presence of subtype switching from primary breast cancer to metastatic breast cancer, along with mutations in the ESR1, PTEN, KAT6A, MYC, MDM4, and AKT3 genes, and copy number losses in the RBI and ARID1A genes.114
Taken together, in breast cancer, the most frequent mutations with potential clinical relevance include alterations in the PIK3CA and ESR1 genes. Frequent alterations with unknown clinical relevance include amplification in the CCND1 and FGFR1 pathway. In addition, rare alterations with potential clinical relevance include those in the BRCA1/2, AKT1, and HER2 genes. In this context, several FDA/EMA approved drugs are available for treating patients with metastatic breast cancer (Table 6, online supplementary Table 1; Figure 4).
CONCLUSIONS
Although the treatment armamentarium for breast cancer has considerably expanded, treatment decision is still based on genetic alterations in the ER, PR, and HER2 genes. However, in the era of precision medicine, several new molecular prognostic and predictive markers are available. In the early luminal cancer setting, genetic signatures help to define and guide treatment decisions in terms of chemotherapy. Furthermore, in patients with early HER2-positive breast cancer, clinical markers like pCR mainly direct decisions in clinical practice. In the advanced setting, molecular profiling can guide treatment decisions (e.g., treating PIK3CA-positive patients with a PIK3CA inhibitor) and can help to identify new targets. The most promising pathways included cyclin-dependent kinase, PIK3CA/mTOR/AKT pathway, BRCA pathway, or overexpression of PD-L1 in patients with TNBC. Further investigation is necessary to standardize the diagnostic and therapeutic approach in breast cancer patients. In the future, every breast cancer patient will have a molecular profile of their tumor to tailor treatment and improve the outcome and toxicity.
TAKE-HOME-MESSAGES
-
In the management of breast cancer, a morphologically and genetically heterogeneous disease, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) are important predictive factors of response to a given therapy in both, the adjuvant and metastatic setting.
-
The recent advancements of molecular profiling methods like next-generation sequencing (NGS), proteomics, and immune profiling have enabled the identification of different actionable targets including alterations in the PI3KCA, ESR1 and BRCA1/2 genes as well as programmed death-ligand 1 (PD-L1) expression. Novel targeted therapies have been investigated in clinical trials, with promising results in specific molecular subtypes.
-
Several international programs, including AURORA from the Breast International Group (BIG), have been launched to improve our understanding of the pathogenesis of breast cancer and deepen our insights into the molecular treatment of the disease.
COI
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors contributed to and approved the final manuscript.
Online supplementary Table 1.
Available from:
https://cdn.healthbook.network/wp-content/uploads/2020/10/table6.jpg.




.png)


.png)





.png)


.png)
