INTRODUCTION
CURRENT TREATMENTS OF NEWLY DIAGNOSED ADVANCED-STAGE OVARIAN CANCER
Approximately 75% of patients have FIGO stage III (a disease that has spread throughout the peritoneal cavity or that involves extraperitoneal lymph nodes) or FIGO stage IV (disease spread to more distant sites) disease at diagnosis.1 The standard treatment of epithelial ovarian cancer (EOC) usually consists of a combination of surgery and neo-/adjuvant chemotherapy. In the advanced stage, bevacizumab, a monoclonal antibody, is added to chemotherapy and used as maintenance therapy. Recently, PARP-inhibitors in BRCA-mutated as well as in BRCA wild-type patients are standard therapies.2–4
Surgical staging and cytoreduction, followed by adjuvant chemotherapy, are regarded as a standard treatment option for most women with EOC. Optimal cytoreductive surgery, e.g., no macroscopic tumor left, is increasing the likelihood that chemotherapy will result in long-term disease-free survival.5 Neoadjuvant chemotherapy (NACT) prior to definitive surgery is an alternative option in selected patients.6
RELATED OVARIAN CANCER
Despite initial therapy, the majority of women with advanced-stage ovarian cancer will, unfortunately, relapse and require further treatment. The likelihood of recurrence depends on many factors, including the stage at initial diagnosis, the success of initial surgical cytoreduction, the velocity of CA-125 decline, and progression-free interval after primary therapy. However, a predictive marker for recurrence has not been prospectively verified.
The management of the relapsed disease is based upon these factors. Women with recurrent EOC should be carefully considered for both secondary cytoreduction and second-line chemotherapy. The relapsed disease varies from isolated tumor sites to peritoneal carcinomatosis. Extra-abdominal sites of recurrence are uncommon. Most patients with recurrent ovarian cancer are treated with systemic therapy alone. Surgical treatment is foreseen for a selected group of patients (e.g., limited disease and eligible for platinum therapy) and a remaining matter of debate. Study results are conflicting.7–13 The standard chemotherapy includes carboplatin in combination with either paclitaxel or gemcitabine or pegylated liposomal doxorubicin (PLD), with the option of adding bevacizumab.14–16
If ovarian cancer is no longer eligible for platinum-based regimens, only a few therapy options remain. Patients who are not eligible for platinum agents due to hypersensitivity reactions or platinum resistance should be offered treatment in clinical trials. Single-agent treatment includes topotecan, PLD, gemcitabine, paclitaxel, or etoposide.17,18 The addition of bevacizumab can be considered even in patients who were exposed to it in an earlier line.19
Despite all efforts and tremendous research, overall survival rates in advanced stage EOC remain poor. Especially in platinum-resistant disease, therapy options are limited. Tumor- targeted drug delivery systems have emerged as a promising strategy in cancer treatment. In recent years, many receptors have been identified as being overexpressed on cancer cells such as human epidermal growth factor receptor 2 (Her-2) in breast cancer or prostate-specific membrane antigen (PSMA) and can be used as oncological targets for individual therapy.20
ROLE OF FOLIC ACID IN THE CELL CYCLE
Folic acid is the precursor of the active co-enzyme tetrahydrofolic acid (THF). THF has a central function in single carbon metabolism. THF acts particularly as a supplier of methyl-, methylene- and formyl groups and is involved in the synthesis of purine bases and deoxythymidine monophosphate (dTMP), which are essential for DNA replication. Because of its involvement in the synthesis of DNA building blocks, folic acid plays a crucial role, particularly in pregnancy and in rapidly dividing cells (e.g., bone marrow or tumor cells).21
Folic acid is essential for the human organism and cannot be produced intrinsically. The recommended daily dose is 200 µg.22 In view of additional effects, 600 µg was previously recommended for healthy adults for the prevention of atherosclerosis and 800 µg for pregnant women and breastfeeding mothers. The daily intake of more than 1,000 µg folic acid has no additional health effects.23
In embryonic development, folic acid deficiency can cause neural tube defects such as spina bifida or anencephaly.24 A deficiency of folic acid in adults causes megaloblastic macrocytic anemia. The co-responsibility of folic acid for cell maturation and differentiation is currently widely investigated.
ROLE OF FRα IN PHYSIOLOGY AND PATHOLOGY OF OVARIAN TISSUE
Folate receptors consist of four glycoproteins α-δ (35-40 kDa), encoded by FOL1-4. FRα and FRβ are attached to the cell membrane, whereas FRγ is free soluble.25 FRα was subsequently found to be a cancer-associated antigen and was first cloned in 1991.26 FRα is expressed in some non-malignant tissue in lower levels (Figure 1), but it is far more often found on tumor tissues making it an interesting target possibility. FRα plays a crucial role in early embryogenesis. Knock-out mice lacking the Folr1 folate receptor showed this through their inability to reproduce due to embryogenesis failure.27 Supplementation of folic acid is an easy and cost-effective way of preventing neural tube defects and is recommended by all leading obstetric guidelines.
In adult organisms, FRα might have a less important role in healthy tissue due to its limited expression and less efficient folate uptake compared to other transporters.29 In pre-clinical models, FRα seems to have a role in cellular migration and invasion and is associated with tumor progression.30 Similarly, FRα-knockdown in ovarian cancer cells results in impaired cell division, migration, and invasion.31 Levels of FRα expression are different across the multiple subtypes of ovarian cancer from high expression in high-grade serous (76%) to lower expression in clear-cell ovarian cancer (32%).32
OPTIONS FOR TARGETING FRα IN ONCOLOGY
Folate receptor α has been considered an interesting target in anti-cancer treatment for several years. This receptor isoform seems to have a minimal physiological role in the adult body and is overexpressed by a variety of different cancer types, mainly ovarian cancer, lung cancer, and triple-negative breast cancer. It is also highly affine for non-physiological substrates such as folic acid, making the development of folic acid conjugates possible. FRα is currently studied in multiple aspects of detecting and targeting tumor cells.
IMAGING
As described above, surgery remains one of the mainstays of ovarian cancer treatment. Therefore, efforts have been made to make malignant lesions more visible for the surgeon, both pre- and intraoperatively. A promising diagnostic option is the FRα-targeted contrast-enhanced magnetic resonance imaging (MRI). There have been investigations with breast cancer cell lines and xenograft with promising results.33 However, these procedures are still under evaluation and not yet readily available. Radiolabelled folate derivatives have also been widely studied. The first results of 111In-diethylenetriaminepentaacetic acid-folate showed promising results in whole-body single-photon emission computed tomography (SPECT) of women with suspicious adnexal masses. The radiotracer was found to concentrate in all malignant lesions with high sensitivity for the detection of malignant lesions when combined with other radiographic methods.34
A cheaper and easier approach for imaging FRα was to produce a peptide derivate of etarfolatide conjugated with 99mTc.35 (Figure 2) This agent showed promising results in pre-clinical studies and is further investigated. Tumor uptake of 99mTc-etarfolatide has been studied as a predictive marker in platinum-resistant ovarian cancer. A positive uptake was associated with improved median progression-free survival (PFS) when treated with vintafolide (folic acid-desacetyl-vinblastine) plus pegylated liposomal doxorubicin (PLD) vs. PLD plus placebo. This could be demonstrated in patients with detectable 10-100% FRα expression but not in those with 0% FRα expression.35,36
Recently, a study has been published using 18F-AzaFol as a first-in-human folate receptor PET-tracer with a favorable safety profile and dosimetric properties in patients with non-small-cell lung cancer.37 In order to improve the detection of tumor cells intraoperatively, fluorescent probes were linked to folates. A folate-conjugated derivate of fluorescein isothiocyanate (Folate-FITC/ EC17) was successfully tested intraoperatively and lead to the detection and resection of ovarian cancer lesions otherwise not detected. However, autofluorescence at certain wavelengths facilitates false-positive outcomes. Therefore, advancing technology could contribute significantly to improving operative results.38,39
KEY TRIALS IN TARGETING FRα
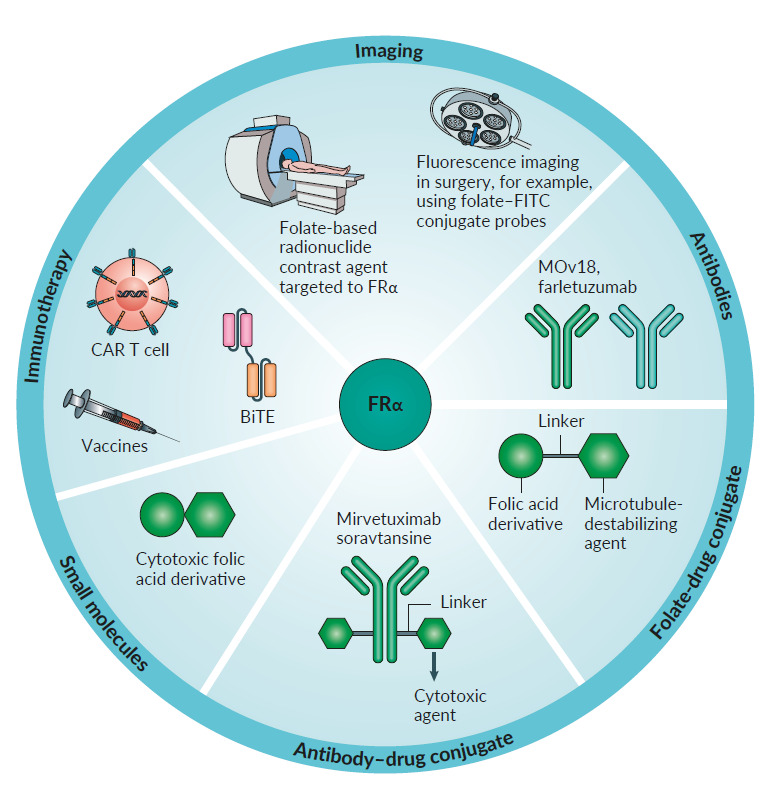
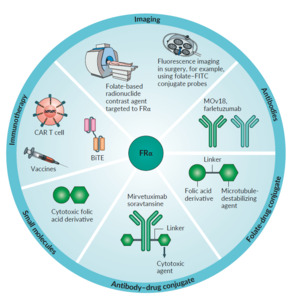
Many potential drugs and methods targeting FRα in patients are currently under investigation in several phase II and III trials, including monoclonal antibodies, folate-drug conjugates, small molecules, vaccines, and CAR T-cells.
Antibodies
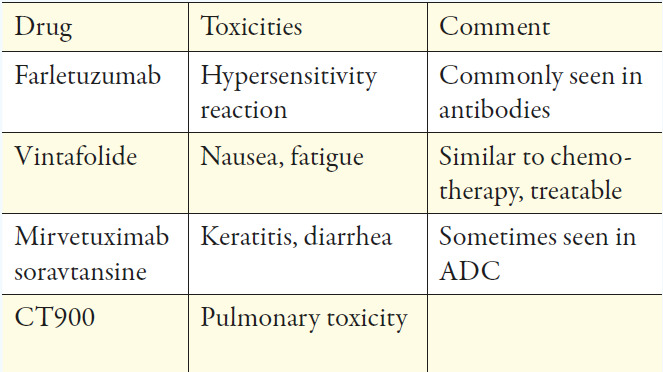
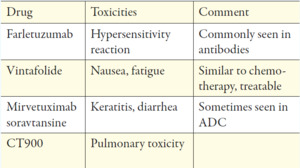
Farletuzumab seemed to be the most promising antibody as it is a fully-humanized IgG1-antibody that targets FRα and exerts its activity against FRα positive cancer cells in several ways. Half-life is approximately 120h, which allows weekly dosing.40 After encouraging results in phase I and II trials, it failed its primary endpoint of PFS in a phase III trial when combined, at different doses, with platinum/taxane versus platinum/taxane and placebo in relapsed ovarian cancer.41 However, in this trial, patients were not stratified by the expression of FRα in tumor tissue, which might be crucial. The previously described 18F-AzaFol PET-tracer could provide an opportunity for a non-invasive way to detect FRα-positive disease.
MOv18 was initially developed as a mouse immunoglobulin G (IgG) antibody, where its radiolabelled form was used to assess the feasibility of radio-immunoscintigraphy. However, IgG- antibodies promote anti-mouse antibodies in humans, which could lead to an anaphylactic reaction. The effects of MOv18 IgG have been evaluated in several early phase clinical trials and proved to be a safe and valuable option to provide images of ovarian cancer lesions. Despite this, further development has not been pursued.42 To avoid anaphylactic reactions, a new MOv18 immunoglobulin E (IgE) antibody was developed and is currently investigated in phase I clinical trial (NCT 02546921).
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are very promising and usually well-tolerated treatment options in targeted therapy. They consist of a cytotoxic payload conjugated to an antibody directed against a tumor-associated antigen. ADCs exert their activity by selective binding of the antibody to tumor tissue internalization and lysosomal degradation and release of the cytotoxic payload, leading to cytotoxic cell death.
Mirvetuximab soravtansine is an ADC consisting of the cytotoxic agent, maytansinoid DM4, linked to a humanized anti-FRα-monoclonal antibody; the approximate half-life is 116-139h and is administered intravenously (IV) every 3 weeks. Pre-clinical and early clinical data were promising and prompted the FORWARD I, phase III trial. Patients with FRα-positive platinum-resistant advanced-stage epithelial ovarian, primary peritoneal, or fallopian tube cancers were randomized to receive chemotherapy (paclitaxel, PLD, or topotecan) or mirvetuximab soravtansine. Unfortunately, the trial failed to meet its primary endpoint on PFS. However, pre-planned analysis of the subgroup with high FRα expression revealed a small but significant improvement of PFS favoring the experimental arm.43 A repeat phase III trial is currently recruiting patients (NCT04209855) using an optimized immuno-histochemical test, measuring both the intensity and location of cellular staining. Multiple early phase clinical trials combining mirvetuximab soravtansine with immunotherapies, other targeted agents, or conventional chemotherapy are currently ongoing. The ADC, MORAb-202, which combines farletuzumab and eribulin, showed potent antitumor activity in cancer cell lines and xenograft models. A phase I trial is currently recruiting (NCT03386942) patients with solid tumors and will be completed in December 2021.
Folate-drug conjugates
A cytotoxic drug is bound to folate via a cleavable linker and is internalized via the FRα receptor via endocytosis and released in the endosome (Figure 3).35,44 A representative of this class is the previously described vintafolide (formerly known as EC145). After promising results in pre-clinical and phase I trials, vintafolide was investigated in the PRECEDENT phase II trial, revealing a benefit when combined with PLD vs. PLD doxorubicin with placebo in platinum-resistant ovarian cancer. However, grade 3 or 4 adverse events were seen in 76% vs. 54% of patients. Furthermore, its short half-life of 20-30 minutes necessitates a 3-times-per-week administration every second week, making it inconvenient for both patients and clinicians.
Furthermore, a phase III trial (PROCEED) was suspended after a prespecified interim futility analysis revealed a lack of benefit in PFS (NCT 01170650).35 Vintafolide is also of interest in FRα positive non-small-cell-lung cancer (NSCLC) and has been studied in a phase II trial (TARGET), showing improvement of objective response rate (ORR) and PFS for the combination of docetaxel plus vintafolide vs. single-agent docetaxel or vintafolide.46 Other agents, such as folate conjugated epothilone, showed no objective responses in phase I/IIa trials and were therefore not further developed, whereas others are currently under investigation.47,48
Thymidylate Synthase Inhibitors
Thymidylate Synthase Inhibitors (TSI) is another possible option for targeting the FRα-receptor of ovarian cancer. TSIs are small molecules that inhibit the thymidylate synthase enzyme. This inhibition prevents C5 methylation from deoxyuridine monophosphate (dUMP), thereby inhibiting the synthesis of deoxythymidine monophosphate (dTMP). Representatives of TSI were earlier studied in the therapy of colorectal cancer, known as quinazolide-derivates. One agent of this drug class, called CT900/BGC945/ONX-0801, is currently investigated in a phase I trial (NCT 02360345) in patients with FRα expressing solid tumors and is estimated to be completed in August 2020.49
CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cell therapy is promising due to the differential expression of FRα-receptor on the membranes of ovarian cancer cells. These T-cells have been genetically engineered to produce an artificial T-cell receptor for use in immunotherapy. Chimeric antigen receptors (CARs) are receptor proteins modified to enable T-cells to target a specific protein. CAR T-cells are engineered to be specific to an antigen expressed on a tumor that is not expressed on healthy cells. Therefore, FRα, with its limited expression in healthy tissue, seems to be an excellent target. Engineered CAR T-cells containing an FRα-specific single-chain variable fragment (scFv; MOv19), coupled to the T-cell receptor chain CD3ζ, in combination with a CD137 (4-1BB) costimulatory motif, were successful in inducing tumor regression in pre-clinical models.50–52 These CAR T-cells are being tested in a phase I trial that is currently recruiting patients with ovarian or fallopian tube cancers, or primary peritoneal carcinoma (NCT03585764) in the US with an estimated Completion Date in October 2020.
CONCLUSION AND FUTURE PERSPECTIVE
The biological rationale for targeting FRα in cancer treatment appears to be appealing, though not all treatment approaches were successful. This raises the question: did we entirely understand targeting FRα? Using FRα targeted imaging agents is promising in making occult tumor sites visible, and thus improving the pre- and intraoperative planning of an optimal resection. The lack of single-agent activity of some of the known agents, such as farletuzumab or vintafolide, likely reflects the non-oncogenic properties of FRα, which permits cancer cells to grow even when FRα is effectively targeted by the antibody.53 Mirvetuximab soravtansine showed limited single-agent activity and likely failed to meet their primary endpoint in phase III trial due to insufficient biomarker stratification. Toxicities were generally manageable and quite comparable with other substances of this drug class.28 Although the available data of phase III trials in targeting FRα may not be successful enough to be used routinely, there are still many promising trials ongoing, which hopefully evolve further knowledge about the substances and where to use it best. For those agents showing single-agent activity, randomized studies of biomarker-stratified cohorts are eagerly awaited, especially in diseases with limited standard treatment options such as platinum-ineligible ovarian cancer.
TAKE-HOME MESSAGES
-
Folate receptor α (FRα) overexpression is seen in ovarian, breast, and lung cancer.
-
Antibodies, as well as antibody-drug conjugates (ADC), are currently being investigated.
-
Lessons have been learned from recent trials to optimize linker technologies in targeting FRα.
-
FRα is a promising biomarker in fluorescence, positron emission tomography (PET), and single-photon emission computed tomography (SPECT) imaging.
-
There may also be a therapeutic option for radionuclides.
COI
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors contributed to and approved the final manuscript.
_and_concordant_expression_of_fr_in_non-mal.png)

_and_concordant_expression_of_fr_in_non-mal.png)