INTRODUCTION
While the interest in precision oncology is constantly increasing since several years and remarkable progress has been made in molecular profiling of most cancer types as well as the development of targeted therapies, gender aspects in oncology are only beginning to gain attention, with the ESMO Workshop “Gender medicine and Oncology” held in 2018, representing an important step.1 This lag is surprising given that a patient’s sex is an important modulator of his disease risk and therapy response, just as age, ethnicity, lifestyle, organ function, and germline and somatic genetic variability.2,3
According to the definition of the WHO, (https://www.euro.who.int/en/health-topics/health-determinants/gender/gender-definitions) “gender” describes those characteristics of women and men that are largely socially created, while “sex” encompasses those that are biologically determined. Gender medicine, or sex and gender-sensitive medicine (SGSM) is an innovative approach to the practice of medicine, which postulates that biological sex differences as well as gender identity impact health and disease, and that these differences may have implications for prevention, screening, diagnosis, and treatment of diseases.1,4 Its ultimate goal is to learn from these differences (or the absence thereof) and improve care and treatment for both men and women. The field of SGSM has gained momentum in recent years and provoked a rethinking of the way scientific experiments, as well as clinical trials, are conducted.
Epidemiological studies show important sex differences in cancer susceptibility and survival. Overall, women have reduced risk and better outcomes than men in a wide range of cancer types.5,6 Interestingly, this trend is independent of ethnicity and exists even in children.7,8 Historically, the generally higher cancer risk in the male population was attributed to stronger exposure to environmental or workplace chemicals and carcinogens, diet, and risk behaviors such as smoking and alcohol abuse. However, even after normalization for these risk factors, women still are less prone to cancer than men, pointing to the presence of protective biological factors in women.9,10 At the same time, although overall insufficiently studied, women are more susceptible to acute hematological and/or non-hematological toxicity, such as mucositis, nausea, and emesis and alopecia of different chemotherapy regimens.2 Notably, higher toxicity rates are also found in girls treated for acute lymphoblastic leukemia.11 Besides inherent differences in tumor biology, multiple factors such as differences in body composition and drug metabolism might contribute to the higher drug toxicity in women. In this review, we will focus on melanoma as a prime example of sex and gender differences in disease risk and outcome and discuss their implications for clinical trial design and drug dosing.
MELANOMA AS A BENCHMARK FOR SEX AND GENDER DIFFERENCES IN ONCOLOGY
Melanoma is prime cancer for investigating sex and gender-related differences in susceptibility and outcome. Men are less likely to self-detect melanomas, have a lower awareness of skin cancer risk, and therefore, are less likely to engage in preventive behavior than women, resulting in diagnostic delay.1 Melanoma in men are likely to be diagnosed when thicker, at an older age, and at a higher AJCC stage.12,13 The analysis of over 11’000 melanoma patients included in the Munich Cancer Registry has shown that women have smaller lesions located mostly on the lower extremities, while men more often have larger lesions located primarily on the trunk.13 Therefore, differences in behavior and clothing choices impact the initial presentation of melanoma.
Men show 15-30% poorer survival rates across all ages than women.14 This is observed in loco-regional melanoma as well as metastatic disease and is consistent across different prognostic subgroups and persists after adjusting for possible confounders such as tumor thickness and localization.14,15 Women have a lower risk of disease progression and have a lower propensity for lymph node and visceral metastases.13 The reasons for this gender gap are not well understood. Contrary to the previous concept, which restricted hormone dependency to cancers of the reproductive tract, accumulating data suggests that hormone levels and receptor expression play a role in the etiology of melanoma as well as other non-sex related cancers such as lung adenocarcinoma and colorectal cancer.16,17 Estrogen receptor β (ERβ) is expressed in melanoma and correlates negatively with Breslow thickness.18 The role of androgens has been investigated in melanoma cells and surrounding stroma. Decreased androgen receptor (AR) expression in primary human dermal fibroblasts (HDFs) induces early steps of cancer-associated fibroblast activation and, in an orthotopic model of skin cancer, HDFs with AR loss increase tumorigenicity of melanoma cells.19 Genetic and pharmacological suppression of AR activity in a large panel of melanoma cells, derived from both male and female patients, suppresses proliferation and self-renewal potential resulting in reduced tumorigenesis in mouse models associated with macrophage infiltration and cytotoxic T cell activation.20 Moreover, AR seems to play an essential role in the maintenance of genome integrity: in both cultured melanoma cells and tumors, loss of AR activity leads to chromosomal DNA breakage, leakage into the cytoplasm, and stimulator of interferon genes (STING) activation.20
Consistent with the role of sex hormones in melanoma development, young age at first childbearing and multiparity reduce melanoma risk.20 Moreover, melanoma is the most common malignancy type diagnosed during pregnancy.21,22 Hormonal changes during pregnancy might affect melanoma development and outcome, although this is still a matter of debate due to conflicting results from various cohort studies.23 Besides the potential impact of sex hormone signaling, differences in oxidative stress neutralization, and the immunomodulatory effects of vitamin D might contribute to the survival advantage of women.24,25
The introduction of immune checkpoint inhibitors targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1), which improve recognition and killing of cancer cells by the immune system in 2011, has revolutionized the treatment of the disease with long term remission in a considerable percentage of patients.26 Melanoma is a strongly immunogenic cancer due to a high mutation burden that results in the generation of multiple neoepitopes.27 Melanomas arising in men exhibit a higher mutation burden compared to those found in women.28,29 This is potentially due to the stronger ultraviolet light-induced immunosuppression and the presence of fewer tumor-associated antigen-specific circulating CD4 T cells in men, which allows melanoma cells to escape the immune system and accumulate mutations.30,31
Pooled analyses of clinical trial data point to sex disparities in the efficacy of immunotherapies.29,32 Female sex has been suggested as a negative predictive factor for the response of melanoma patients to anti-PD1-therapy.33 One explanation for this finding might be the paucity of partially exhausted PD-1high/CTLA-4–positive CD8 cells associated with response to combined checkpoint inhibition in women, while the hormone-mediated mechanism might also be important.34 A recent comprehensive analysis of data from The Cancer Genome Atlas (TCGA) has confirmed sex bias in immune features such as tumor mutation burden, immune cell infiltration, and expression of immune checkpoints in multiple cancer types, including melanoma.29 In addition, it has been reported that women are at higher risk of experiencing immune-related adverse events during anti-PD1 therapy.35
In the absence of pre-planned subgroup analyses according to sex from large clinical trials, no definitive conclusions on therapy efficacy and toxicity can be drawn yet.
EXAMPLES OF SEX DIFFERENCES IN GASTROINTESTINAL CANCERS
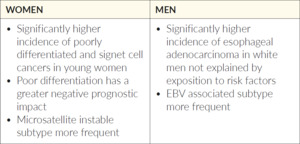
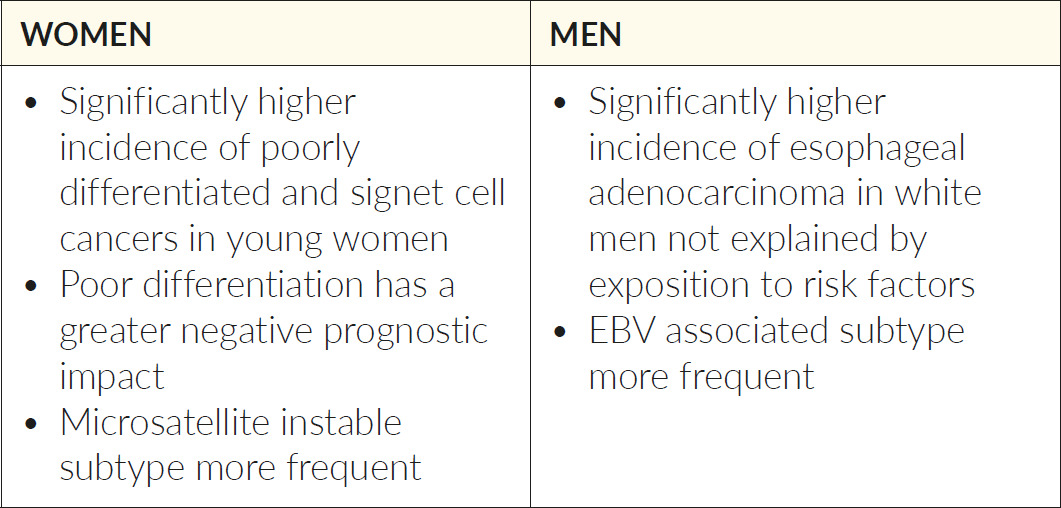
In gastrointestinal cancers, multiple examples of sex differences in tumor biology cancer can be found as well. In both colorectal and gastric cancer, molecular subtypes are not equally distributed between men and women. While in colorectal cancer, the consensus molecular subtype 1 is more frequent in women, in gastric cancer, microsatellite instable tumors are more frequent in women, and Epstein-Barr virus (EBV)-associated tumors are more frequent in men.36,37 In colorectal cancer, tumors arising in women are more likely to harbor BRAF-mutations (10% vs 6.5%) and are more often right-sided, while tumors arising in men are more often located in the rectum (26 vs 32%).38 In gastric cancers, women have significantly more often poorly differentiated (42 vs 34%) or signet ring cell carcinomas (28 vs 16%) in a large retrospective series from Korea.39 This observation has been confirmed by other authors from different countries. In fact, sex-biased gene expression signatures in actionable genes have been detected in different types of cancer, and support the concept of “sexual dimorphism in cancer” (Table 1 and 2).19,37,39–42
REVISITING CLINICAL TRIAL DESIGN IN THE ERA OF GENDER MEDICINE
In the era of precision oncology and gender medicine, clinical trial design needs to be revisited. The progress in precision medicine and the development of biomarkers have led to the generation of innovative trial strategies. In basket trials, targeted therapy is tested on multiple diseases with a common molecular alteration, while umbrella trials evaluate multiple targeted drugs for a single cancer type that is stratified into molecular subtypes. Yet, among other reasons, the insufficient inclusion of women in clinical trials remains an important issue that hampers the design of appropriately powered large clinical trials, which would allow meaningful subgroup analyses according to sex.
The underrepresentation of women in clinical trials has historical reasons. The regulation of the participation to clinical trials started following the scandals of drug-induced birth defects related to diethylstilbestrol and thalidomide. Diethylstilbestrol was prescribed to prevent abortions and premature birth and was later associated with higher rate of these pathologies and cervical and vaginal cancers in the offspring. Thalidomide was used from 1957 on as a sedative and to treat pregnancy-associated nausea and was discovered to cause the malformation of the limbs (phocomelia), leading to its retraction from the market in 1961. In 1977 the Food and Drug Agency (FDA) released the “General Considerations for Clinical Evaluation of Drugs”. It recommended that women of childbearing potential should not participate in the early phase (I and II) clinical trials until sufficient data on drug safety had been obtained. This led, however, practically to the exclusion of women in general from clinical trials, not only early phases. Although based on a good intention, the effects of this guideline were rather detrimental than beneficial, leading to the underrepresentation of women in clinical trials until today. It was criticized by advocacy groups to interfere with the right of women to decide autonomously whether or not to participate in clinical trials, to violate the principle of informed consent, and deny women access to potential therapies.41
As a reaction, in 1993, the FDA issued the “Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs” guidelines, which allowed the inclusion of females into phase I and II studies and endorsed analysis of data on sex differences for efficacy, toxicity, and pharmacokinetics.42 However, according to the General Accounting Office (GAO) report of 2001, although the inclusion of women in studies is adequate, the majority of participants in early phase trials are still male, and sex differences in response to drugs remain insufficiently examined.43 Indeed, an analysis of the FDA approved phase I trials between 2006 and 2007 showed that over one-third of them had only male participants, and in studies with both sexes, women accounted for about 30%.44 This is possibly due to the challenges related to regulations for birth control for women, such as two methods of contraception and the use of oral contraceptives for 3 months prior to entry into the study.
In contrast to the FDA, the European Medicines Agency (EMEA) concluded, based on an analysis of the marketing applications between 2000 and 2003, that the current guidelines cover the special needs of women and found that women are appropriately represented in clinical trials.45
In our view, there is still space for improvement for clinical trial design and reporting of sex differences. The National Institute of Health (NIH) requires all clinical studies funded by the NIH to include women and minorities and phase III trials to be designed and powered to facilitate gender analysis. Despite these regulations, an analysis of cardiovascular studies found that only 51% of NIH funded trials and 22% of non-NIH funded trials reported subgroup analyses by sex.46 Data on reporting on subgroups in other fields, such as oncology, is largely missing.
Systematic investigation and reporting of the presence or absence of sex differences are critical to the improvement of patient care in oncology. In order to avoid that clinically relevant differences remain undetected, the relationship between drug dose, response, and toxicity should be evaluated separately in men and women using data from large clinical trials and pooled analyses.2 The current process of drug development is not designed to define potentially different optimal doses for women and men. This points to another important issue that needs revisiting – the dosing of anticytotoxic drugs according to the body surface area (BSA).
REVISITING CURRENT BSA BASED DOSING
Apart from differences in tumor biology, sex differences in drug effects are well known and have been described several years ago.47 A recent review of sex differences in the pharmacokinetics of anticancer drugs found differences of about 20% in around 20% of population pharmacokinetic studies.1 5-FU is only one example of a drug with a significantly higher clearance in men.48 This difference translates into statistically significant and clinically relevant higher hematological (especially leucopenia and neutropenia) and non-hematological toxicities (e.g., diarrhea and mucositis) in women, which is well documented in several large studies for both adjuvant and palliative chemotherapy for colorectal cancer.39,49,50
One factor known to influence drug metabolism is the sex difference in fat-free body mass. While the metabolically active, fat-free body mass constitutes about 80% of a man’s total body mass, the fat-free body mass of a woman is only 65% of her total fat-free body mass. Importantly, these differences are not taken into account when dosing chemotherapy, according to the body-surface area.1 Computed-Tomography scanning (CT) is a ubiquitously available and precise tool for the estimation of human body composition.51 Multiple studies confirmed the association between sarcopenia and toxicity, for example, in patients treated with capecitabine or sorafenib.52,53 Assessment of body composition by CT scan has not only a significant potential to improve drug dosing, but also provides important prognostic information.
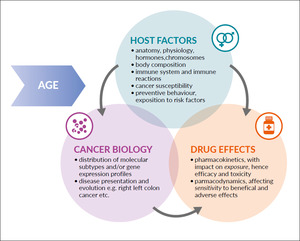
The Sexie-R-CHOP-trial, which prospectively investigated different doses of rituximab in men and women with diffuse large B-cell lymphoma and demonstrated an improved progression-free survival by 32.5% (p=0.039), with a trend for better overall survival and without increased toxicity, demonstrating the feasibility of such trials and representing an example for the potential of rationally designed, sex-specific dose modifications to improve the balance between efficacy and toxicity of anticancer therapies in men and women.54 More such trials, which also taking into consideration the individual patient’s body composition and toxicity as a biomarker for exposure, are required (Figure 1).
CONCLUSIONS
The investigation of sex differences should be performed in a hypothesis-driven fashion rather than being descriptive. There is vast evidence on sex differences in cancer susceptibility and survival as well as drug effects, and the elucidation of underlying biological mechanisms, drug dosing strategies taking into consideration the individual patients’ body composition needs further investigation. In addition to currently and in future potentially available biomarkers, the patients’ sex as an independent modulator of drug efficacy and toxicity merits better understanding and consideration for further individualization of treatments.
TAKE-HOME-MESSAGES
-
Significant sex differences exist in the pharmacology of anticancer drugs.
-
In addition, increasing evidence supports the concept of a “sexual dimorphism in cancer”, and sex-biased gene-expression signatures in different types of solid tumors, have been described.
-
Body surface area (BSA)-based chemotherapy dosing ignores both – sex differences in fat-free – body mass and the large interpatient variability in body composition and needs reconsideration.
-
Especially in diseases or subgroups with significant differences in epidemiology or outcomes, men and women with non-sex-related cancers should be considered as biologically distinct groups of patients, for whom specific treatment approaches merit consideration.
CONFLICT OF INTEREST
BCÖ has no conflict of interest to declare.
ADW has declared to receive personal fees or travel support from BMS, Servier Suisse, Merck, MSD, Bayer, EMD Serono, Lily, Celgene, Shire, Pierre-Fabre, and Pfizer. Non-financial support (travel fees) from Sanofi, AstraZeneca, Abbvie, Ipsen, are all outside the submitted work. She is the coordinating investigator of EORTC-trial 1203, which is supported by an educational grant from Roche to EORTC.
AUTHOR CONTRIBUTIONS
BCÖ and ADW contributed equally to this manuscript.