INTRODUCTION
Leukemia is one of the most common types of cancer in young people, representing 28% of all childhood cancers, 13% of cancers in adolescents, and 3% of all newly diagnosed cancers in females.1 The 5-year survival rates vary between 54% and 94%, depending on the type of leukemia and the patient’s age at the time of diagnosis. The most common types of leukemia in children and adolescents are acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML). However, chronic myeloid leukemia (CML) and chronic lymphocytic leukemia (CLL) can also occur in these age groups.1 Each of these hematological malignancies can adversely affect fertility due to the inherent characteristics of the disease itself, the gonadotoxic potential of specific anti-cancer therapy and the patient’s age at diagnosis.2 Taking all of these aspects into account, female leukemia survivors have significantly compromised chances of subsequent pregnancies.3
Despite the strong potential of anti-cancer therapy to impair fertility, along with the strong impact of infertility on quality of life, the issue of fertility preservation in cancer patients has still not been completely clarified and routinely discussed in clinical practice.4,5 Therefore, the term oncofertility was introduced, representing a new interdisciplinary field at the crossing of oncology and reproductive medicine, to promote fertility preservation in young cancer patients.6 Based on current clinical experience, the oncofertility approach in leukemia is particularly demanding. Therefore, in this article, we focus on the current multidisciplinary challenges to improve fertility preservation outcomes in these patients and encourage communication between hemato-oncologists and reproductive specialists.
GONADOTOXICITY
Mechanisms and risk assessment
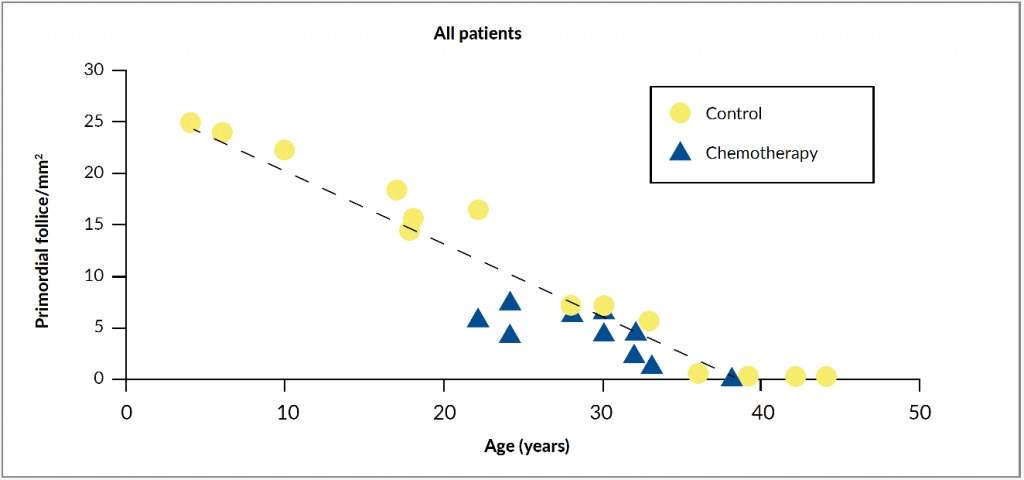
Females are born with around 1 million primordial follicles, and by the time of puberty, only about 400,000 of them remain.7 In each menstruation cycle, multiple follicles enter the maturing pool. Due to the follicle-stimulating hormone (FSH) stimulation, one of them becomes the dominant follicle, which produces estradiol triggering a luteinizing hormone (LH) surge followed by ovulation. The remaining follicles in the maturing pool undergo atresia and apoptosis, leading to a decline in the number of primordial follicles with increasing age.8 This physiological decrease of ovarian reserve can be significantly accelerated by gonadotoxic cancer treatment. Chemotherapy actively targets dividing cells and induces apoptosis in granulosa cells essential for the growing follicles, leading to the destruction of mature ovarian follicles. Moreover, chemotherapy can also cause apoptosis of nongrowing primordial follicles, ovarian atrophy and cortical fibrosis.9 All these processes can result in either premature menopause in case of moderate depletion of primordial follicles, or even in acute ovarian failure in case of almost complete depletion (Figure 1).7
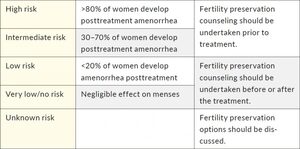
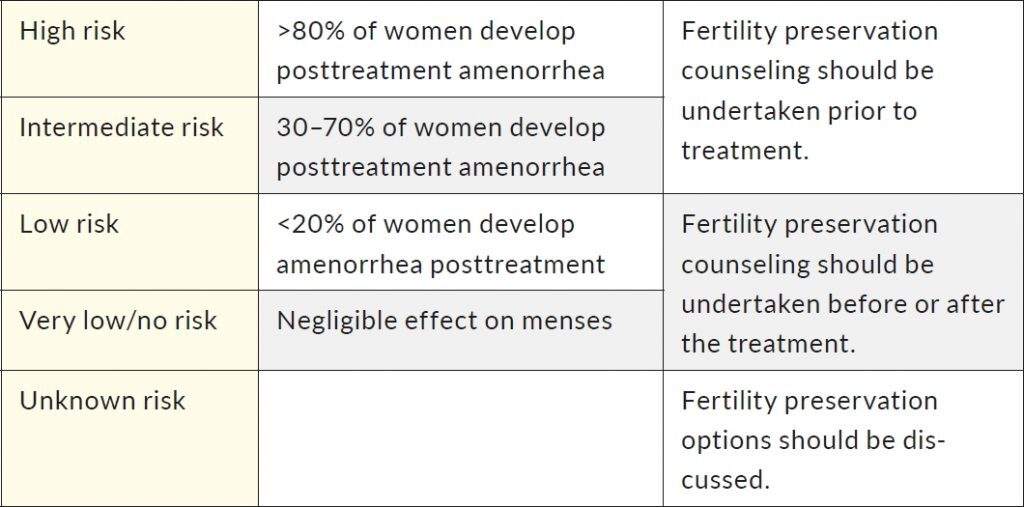
There is still a significant inconsistency in monitoring gonadotoxicity, mostly due to different availability of data in medical records, type of laboratory tests, the variability of follow-up methods, and lack of control groups.11 Additionally, different classifications to assess the risk of gonadotoxicity exist for different agents. One of the most commonly used classifications based on the prevalence of chemotherapy-induced amenorrhea was proposed by the Livestrong organization (Table 1).12
Chemotherapy-induced amenorrhea is typical in cancer survivors, where menstruation usually resumes within two years, however, these women may still have decreased ovarian reserve.13,14 Among other frequently used indicators are pregnancy and birth rates, infertility measured as an inability to conceive after one year of attempting, acute ovarian failure, premature menopause, and gonadal insufficiency.11 In this regard, anti-Müllerian hormone (AMH) very accurately correlates with the antral follicle count (defined as the number of follicles in the ovary measuring 2–10 mm in diameter).15 It can, therefore, predict decreased ovarian reserve earlier than other laboratory markers. It is possible to determine the concentration of AMH in prepubertal as well as postpubertal females.16
Systemic anti-cancer therapy
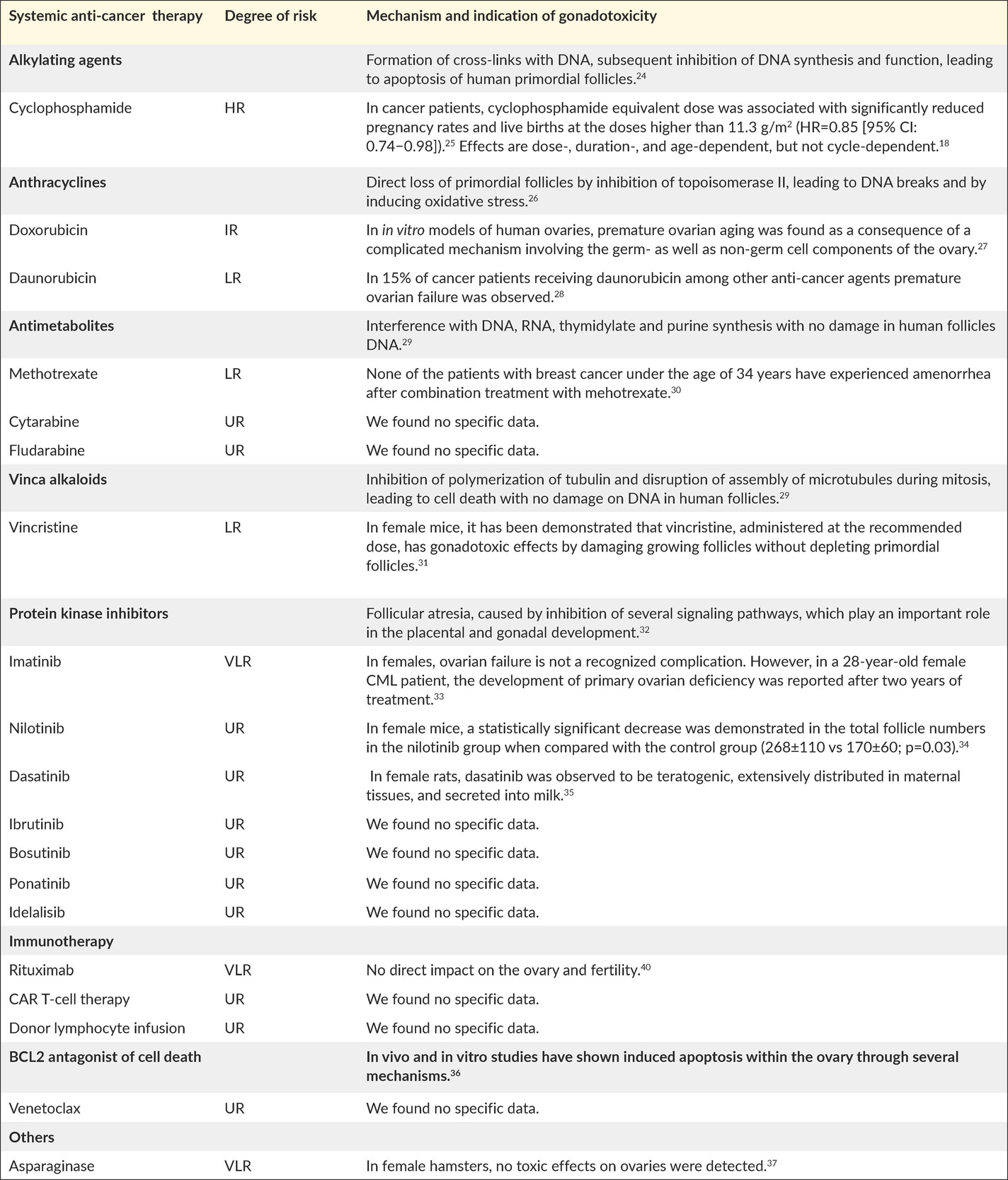
Chemotherapeutic agents can impair fertility by inducing prenatal loss of oogonia, direct loss of primordial follicles, accelerated activation of primordial follicles, follicular atresia, damage to the ovarian stromal tissue or vasculature, and inflammation.17 Importantly, understanding the mechanisms by which cancer therapies cause gonadal impairment may lead to the development of neoadjuvant cytoprotective pharmacotherapy, capable of protecting the ovary without compromising the efficacy of the anti-cancer therapy.18 Table 2 presents standard anti-cancer agents for leukemia in the target age group, and their known gonadotoxic mechanisms.19–23
In general, the reproductive ability of girls and young women treated with conventional chemotherapeutic protocols alone for ALL and AML is mostly protected.38 Low-dose regimens have demonstrated better fertility outcomes in the treatment of Hodgkin lymphoma, showing that those options could also be applied for leukemia patients.39 Less is known of infertility in CLL and CML patients since the gonadotoxic effect of protein kinase inhibitors is less well-established.40 Similarly, no potential risks are known for other immunotherapy strategies, such as chimeric antigen receptor T (CAR T) cell therapy and donor lymphocyte infusions.41 However, patients receiving these treatments are often heavily pretreated, meaning their fertility is likely already impaired by previous regimens. Clearly, a better understanding of the gonadotoxic mechanisms of novel chemotherapeutics and combination therapies is needed not only to protect against iatrogenic depletion of ovarian follicles but also to prevent endocrine disruptions.42
Radiotherapy
Although total body irradiation for hematopoietic stem cell transplantation (HSCT) is rarely used nowadays, cranial radiotherapy for metastatic disease can cause disorders on the hypothalamic-pituitary axis, and pelvic radiotherapy can severely impair ovarian function by causing depletion of germ cells and reduction in the production of sex hormones.43–45 Several radiation oncologists recommend fractionated radiotherapy whenever possible to minimize direct and indirect damage to the ovaries.46–48
FERTILITY PRESERVATION
To date, several different strategies for fertility preservation have been applied to cancer patients.49 The technique selection should depend on the treatment plan, the age, the risks and efficacy, the individual preference of the patient as well as costs and accessibility.46 Nevertheless, the options for the distinct population of females with leukemia are still limited.
Prepubertal girls
Unfortunately, the most conventional methods, such as embryo and oocyte cryopreservation, are not suitable for prepubertal girls due to the inactive hypothalamic-pituitary-ovarian axis, leaving them with only limited options for fertility preservation, such as ovarian tissue cryopreservation, oocyte maturation in vitro, and other novel methods.
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation, followed by autotransplantation, is the most discussed currently available option for prepubertal female cancer survivors, which can restore not only reproductive but also endocrine ovarian function.49 Here, a part of the cortical ovarian tissue is surgically excised, cryopreserved, and transplanted back when the cancer is cured and the pregnancy desired. Corresponding pregnancy rates after ovarian transplantation in postpubertal females are 23–37%, while data on outcomes in prepubertal cryopreserved ovarian tissue is limited to a first successful live birth in 2015.47,48 Furthermore, there is a high risk of ovarian metastases in leukemia, which were found in more than 50% of patients.50 That is why ovarian tissue cryopreservation with autotransplantation should still be considered as an experimental technique in this setting.50 Nevertheless, the first case of delivery in a leukemia patient has been reported, in which they evaluated ovarian tissue for leukemia cells contamination prior to autotransplantation.51 Some authors conclude this procedure should still be performed, considering the progression of novel techniques, such as in vitro maturation and artificial ovary.52–54 However, the patients should be thoroughly informed about the possible risks and the potential futility of this promising method.52
Oocyte maturation in vitro
One step further from ovarian tissue cryopreservation is in vitro maturation of oocytes, which represents a possible alternative to ovarian tissue cryopreservation for cancer patients with a high risk of ovarian metastases.47 In this method, oocytes are extracted either in vivo or ex vivo from the excised ovarian tissue, then cultured in vitro for up to 48 hours before in vitro fertilization (IVF) or cryopreservation.53 Although the success rates after a fresh transfer are comparable to the traditional IVF, the success rates after cryopreservation are still controversial.55–58 The first live birth after in vitro maturation and cryopreservation was in 2020.59 Nevertheless, due to existing controversies, von Wolff et al. (2018) recommended that the method should be limited to women with a very high antral follicle count and without time to perform gonadotropin stimulation.
Experimental methods
One of the most promising novel methods is the artificial ovary.47 This innovative technology attempts to generate mature oocytes ready for IVF through a multiphase ex vivo approach, involving simultaneous ovarian, follicle, and oocyte cultures in vitro.54 Moreover, techniques like in vitro growth of harvested follicles, oocyte generation using ovarian tissue xenografts in animal models, as well as stem cell (e.g., mesenchymal) utilization, might be available in the future with limited ethical dilemmas.60–63
Additionally, understanding of the gonadotoxic mechanisms of commonly used chemotherapeutic agents has enabled the development of preventive adjuvant pharmacotherapy.49 There is now a range of agents attempting to protect the ovary against chemotherapy by preventing direct loss and accelerated activation of primordial follicles, as well as follicular atresia.17 Although still experimental and only tested in animal models, these methods represent the unique opportunity for fertility preservation in prepubertal leukemia patients with a high risk of ovarian tumor cells.49 However, all these regimens still need to be further investigated and developed to be able to protect human ovaries without contralateral damage.
Postpubertal girls and young women
Although the previously mentioned methods can also be used in postpubertal females, there are few more well-established options with better outcomes that are suitable for this patient group. These include embryo and oocyte cryopreservation, hormonal therapies, and alternative methods such as adoption and oocyte donation.
Embryo and oocyte cryopreservation
For decades, the preservation of fertilized oocytes has been the gold standard for infertile postpubertal women. With the introduction of cryopreservation of unfertilized oocytes, the cryopreserving technology has also expanded to infertile postpubertal girls and single women.49 The standard ovulation stimulation protocol, using a combination of gonadotropin-releasing hormone (GnRH) antagonists, gonadotropins, and GnRH agonists, poses minimal risk of ovarian hyperstimulation syndrome (OHSS).64 Stimulation can start at any time during the menstrual cycle. Double stimulation and stimulation directly after ovarian tissue removal are also possible.65–67 In a large retrospective analysis, patients with hematological malignancies had a higher number of mature oocytes retrieved; however, the fertilization rate as well as the number of cycles canceled due to OHSS, lack of response to stimulation, and fertilization failure were comparable with other types of cancer.68 In cancer patients, the pregnancy rate with frozen embryo per patient was 54.5%, with a subsequent live birth rate of 22.72%.69 Comparably, live birth rates in healthy IVF patients were 22–36%. However, there is no specific data of success rate in oocyte cryopreservation for cancer patients, with only 8.36% of vitrified oocytes resulting in pregnancy in healthy IVF population.70
Hormonal therapies
GnRH analogs could be prescribed to preserve fertility. However, their role in protecting ovaries before and during chemotherapy in hematological malignancies is controversial.71–73 The explanation of their potentially protective function depends on the assumption that the downregulation of pituitary gland will decrease the proliferation rate in the ovaries and result in a lower sensitivity to the cytotoxic agents.74 Most meta-analyses provide evidence that temporary ovarian suppression with GnRH analogs during chemotherapy is an effective and safe option to reduce chemotherapy-induced premature ovarian insufficiency. However, its impact on conception remains to be determined.71,72,75–77 Additionally, combination oral contraceptives (COC) represent another hormonal option that may decrease the incidence of ovulation and bleeding during chemotherapy.78 A German retrospective study has shown that the COC group had a reduced risk of amenorrhea compared with the women not receiving COC during chemotherapy.39 Similarly, a systematic review reported a decreased risk of premature ovarian failure in patients receiving COC.79
Alternative methods
If available fertility preservation methods fail or are not undertaken due to specific reasons, there are still some alternative methods such as adoption and oocyte donation. While surrogacy is currently not legal in Switzerland, patients may travel to countries where this procedure is allowed.80 This method is growing in popularity and is called cross-border reproductive care.81
ONCOFERTILITY APPROACH
Current challenges
Hemato-oncologists and reproductive specialists face many challenges in counseling female patients with leukemia about fertility preservation not only due to the anti-cancer treatment but also to the specific patient group and the disease itself.
Urgent treatment
One of the most frequent challenges in the oncofertility approach is the acute onset of leukemia, which often requires urgent treatment. These patients have no time for fertility preservation procedures since the delay of anti-cancer treatment could endanger their life.64,78 Additionally, these patients often present with a poor health condition, including cytopenia, infection, and cardiopulmonary issues, which also makes them ineligible for most fertility preservation procedures.49 Postpubertal girls and women could receive GnRH agonists, and COC after risks of accelerated bone loss and thromboembolic events have been carefully calculated, while there is no alternative for prepubertal girls.78
Prepubertal age
Another common problem is that most female patients with leukemia are prepubertal girls with minimal options for fertility preservation. Fortunately, modern chemotherapeutic regimens result in modest fertility impairment; therefore, their fertility might be preserved even if no unique preservation methods are undertaken.1,9 Nevertheless, some authors suggest that ovarian tissue cryopreservation should still be performed in girls who can undergo laparoscopic surgery. In the future, this procedure might be routinely used due to the advances in technology.78
Unknown gonadotoxic risk
Counseling on infertility and fertility preservation in leukemia patients can be particularly challenging due to uncertainties in ovarian failure as well as of ovarian recovery rates. Furthermore, the gonadotoxic risks of some novel anti-cancer regimens are still unknown. There is also an undefined risk of some fertility preservation methods that are immediately available. Therefore, a question may arise if we can overtreat patients with fertility preservation.78 In this case, a multidisciplinary approach with fertility counseling and predefined criteria for fertility preservation methods may reduce the uncertainty.
High risk of relapse
Most of the modern chemotherapeutic regimens are effective with doses that have a low or very low risk of gonadotoxicity. However, there is a high incidence of relapse in leukemia. In this setting, treatment is intensified, including high-dose chemotherapy and HSCT, which might significantly reduce fertility. Therefore, even after completing therapy, fertility counseling should be undertaken with all patients under the age of 40 years, and further procedures performed depending on the patient’s wish.9
Chronic myeloid leukemia
Although CML represents only 0.4% of all leukemias in females between 15 and 19 years of age, it is especially challenging for fertility management.9 The standard of care probably represents a minimal risk to induce infertility; however, it is recommended to consider oocyte or embryo cryopreservation to achieve a timely predicted pregnancy. Namely, due to the potentially harmful and teratogenic effects of treatment with tyrosine kinase inhibitors, the treatment should be interrupted at the time of pregnancy, if the patient has been in a major molecular response for more than two years. Since natural conception can take a while, IVF can limit the off-therapy time and therefore reduces the risk of relapse.46,72,82,83 During pregnancy, the tyrosine kinase inhibitors could be exchanged for interferon, where some patients with deep molecular response might even stay treatment-free.84
Education of hemato-oncologists
Recent studies have shown that most hemato-oncologists (79%) and HSCT specialists (87%) are aware of gonadotoxic risks of anti-cancer treatment and counsel patients about the possibility of infertility.85,86 Since reproductive health is not their primary concern, it is recommended that hemato-oncologists closely collaborate with reproductive specialists and refer patients to fertility counseling at the time of diagnosis.49,64,78 However, a recent study showed that only 36% of female patients were referred to reproductive specialists.85 A simple discussion about possible risks and benefits has shown substantial improvements in the reduction of stress in patients.87 It is essential that we further promote the multidisciplinary approach, which could reduce stress not only for patients but also for the treating hemato-oncologists, who could, therefore, focus more on anti-cancer treatment.
Costs
The additional financial support offered either by the health insurance or by the state, might help increase the number of fertility preservation procedures in leukemia patients.88 For example, in the United States, only 3–10% of cancer patients decide for fertility preservation. The main reason for not providing the oncofertility approach for all suitable patients is the high financial burden. Conversely, in countries like France, where fertility preservation is a statutory right, they reported that 23% of eligible women with breast cancer had undergone fertility preservation procedures.89 In Switzerland, some assisted reproductive techniques have been covered by insurance since July 2019.90 However, earlier, the costs of fertility preservation in pediatric and adolescent cancer patients were primarily covered by parents and less frequently by health insurance or organizations, such as the Swiss Cancer League.52
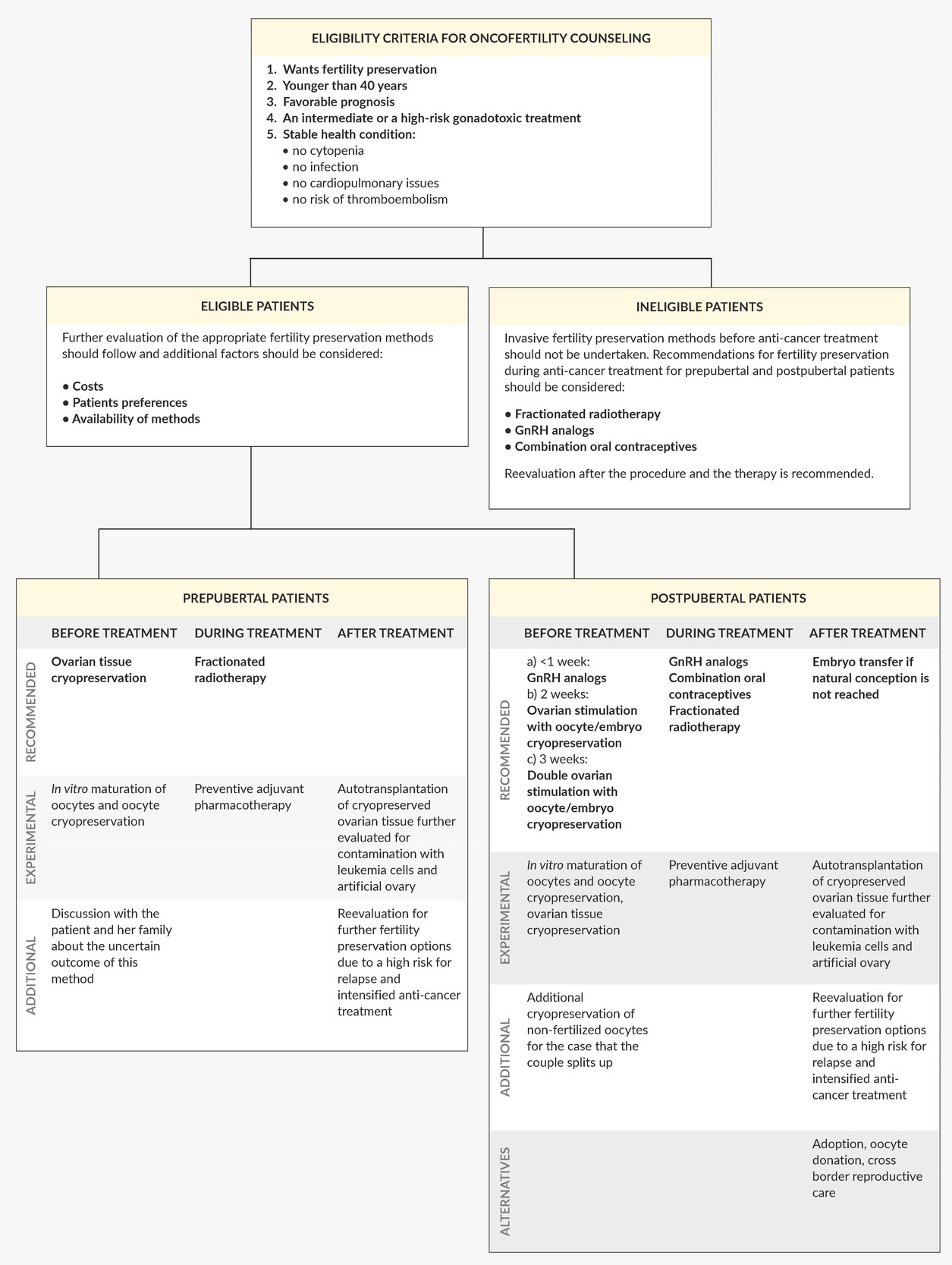
Our model for the oncofertility approach
Numerous guidelines for fertility preservation have been published around the world.46,49,64,78 Taking all their aspects and current challenges into consideration, we have designed a model for the oncofertility approach in females with leukemia (Figure 2). We aimed to help hemato-oncologists better understand the current situation and to facilitate the decisions made by reproductive specialists. Although leukemia patients mostly have limited options for fertility preservation, they must be aware of the gonadotoxic effects of anti-cancer treatment, possibly before the beginning of therapy. Therefore, fertility preservation should be routinely discussed with every patient in the reproductive age, and further referrals to specialized oncofertility centers should be undertaken.
CONCLUSIONS
To mitigate the gonadotoxic risks in girls and young women with leukemia, different fertility preservation methods could be undertaken before, during, and after treatment based on the age of a patient. In order to improve reproductive outcome and quality of life in young cancer survivors, close collaboration between hemato-oncologists with a multidisciplinary oncofertility approach is necessary.
COI
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors contributed to and approved the final manuscript.