INTRODUCTION
Anti-angiogenic treatment for metastatic colorectal cancer (mCRC)
Almost all malignant tumors are partially dependent on angiogenesis for tumor growth. Vascular endothelial growth factors (VEGFs) are key players in angiogenesis and thus, targeting the VEGF pathway in combination with chemotherapy is a common and effective treatment strategy.1 The VEGF family comprises of the cytokines, VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PIGF), and their receptors, VEGFR-1, VEGFR-2, and VEGFR-3.2 Preclinical studies have shown that the VEGF family cytokines induce angiogenic response via interaction with their receptors leading to the activation of multiple pathways.3
The combination of chemotherapy plus bevacizumab, an antibody targeting specifically VEGF-A, is commonly used as the first-line treatment for patients with metastatic colorectal cancer (mCRC).4 However, most mCRC patients treated with bevacizumab ultimately experience disease progression.5 Preclinical evidence suggests that VEGF-B and PIGF can trigger the activation of other angiogenic pathways, which can compensate for the depletion of the VEGF-A levels.6 Thus, targeting a wider range of angiogenic factors could potentially be a more effective strategy in the secondline. However, this needs to be further validated in future clinical studies. Although the mechanism of resistance to bevacizumab in the first-line is still not well known, several studies have shown that the altered expression of various plasma and tissue biomarkers can be predictive of bevacizumab resistance.7,8 In addition, it can also be instrumental in selecting the most effective second-line anti-angiogenic option. In case of patients who progress on first-line bevacizumab therapy, a significant upregulation of various cytokines, like VEGF-A, PIGF, bFGF, HGF and MMP-9, were observed prior to progression on bevacizumab therapy.7,8 It has also been observed that the expression of VEGFR-2, growth factor receptors involved in angiogenesis, is enhanced during treatment with bevacizumab. Based on these elevated expression levels, these cytokines may act as biomarkers of resistance to therapeutic strategies involving anti-angiogenic agents like bevacizumab. In addition, they are also known to be associated with poor clinical outcomes. As anti-VEGF therapy forms an essential part of first- as well as second-line mCRC treatment strategy, identifying biomarkers of resistance to anti-angiogenic agents is of utmost importance.7,9
Options for second-line anti-angiogenic therapy
In the case of mCRC patients, continuing treatment with anti-angiogenic agents after first progression is an option. In the second-line setting, there are three evidence-based alternatives, based on the results of three different phase III trials, i.e. bevacizumab beyond progression (ML18147 trial),10 ramucirumab (RAISE trial),11 and aflibercept (VELOUR trial).12 However, making the optimal choice is challenging due to the different eligibility criteria used in the ML18147 (also known as TML trials), RAISE and VELOUR trials and the lack of head-to-head comparisons of these three anti-angiogenic agents.13,14
The phase III ML18147 trial demonstrated the superiority of bevacizumab plus chemotherapy in comparison with chemotherapy alone in the second-line treatment of mCRC patients (median overall survival [OS]: 11.2 vs 9.8 months HR: 0.81 [95% CI: 0.69−0.94]; p=0.0062).10 It is noteworthy that in this study, the chemotherapy backbone was switched between oxaliplatin-based and irinotecan-based chemotherapy in the second-line setting based on the first-line regimen used.10 In the phase III RAISE trial, ramucirumab, another anti-angiogenic agent, which binds to VEGF-D, was investigated in combination with chemotherapy in mCRC patients who progressed during or after treatment with bevacizumab plus chemotherapy. The results of this study showed that ramucirumab plus FOLFIRI (folinic acid, fluorouracil and irinotecan) led to a significant improvement in the median OS (mOS: 13.3 vs 11.7 months HR: 0.844 [95% CI: 0.73−0.97] log-rank p=0.0219).11 Similarly, the phase III VELOUR trial demonstrated that the addition of aflibercept to FOLFIRI provided a statistically significant benefit in terms of OS (13.50 vs 12.06 months HR: 0.817 [95.34% CI: 0.713−0.937], p=0.0032; intention-to-treat [ITT] population) and progression-free survival (PFS) (6.90 vs 4.67 months HR: 0.758 [99.99% CI: 0.578−0.995], p=0.00007; ITT population) in oxaliplatin-pretreated mCRC patients, including those treated with bevacizumab (and other prespecified subgroups as well).
Several studies have shown that the altered expression levels of various plasma and tissue biomarkers can be instrumental in selecting the most effective second-line anti-angiogenic option. For example, high VEGF-D levels after progression on bevacizumab were shown to correlate with better response to ramucirumab in the RAISE trial.14,15 In addition, certain patient characteristics can also predict response to therapy. For instance, subgroup analyses in the ML18147 and VELOUR trials suggested that short progression-free survival (PFS) in first-line therapy with bevacizumab can be used to select the optimal anti-angiogenic agent in the second-line setting.16
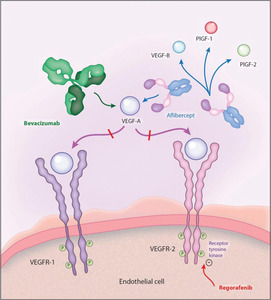
Aflibercept is an anti-angiogenic protein, comprising of key domains from the human VEGFR-1 and -2, fused with the Fc portion of the human immunoglobulin G. Unlike other anti-VEGF agents like bevacizumab (specific for VEGF-A) and ramucirumab (specific for VEGFR-2), aflibercept exhibits a wider binding range by blocking the interaction of different VEGF cytokines, including VEGF-A, VEGF-B, and PlGF with their receptors (Figure 1).13,16,17 Preclinical studies have shown that aflibercept is superior to bevacizumab irrespective of prior treatment status (i.e. with or without pretreatment with bevacizumab) by inducing a greater tumor response in mCRC animal models.4
PLASMA BIOMARKERS
The current anti-angiogenic therapy is primarily based on blocking the VEGF-A pathway. However, acquired resistance to anti-VEGF-A therapy remains a major challenge.7,19
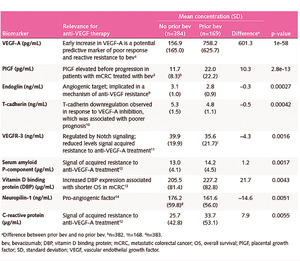
The results of the VELOUR study showed that aflibercept has consistent and good efficacy in mCRC patients pretreated with oxaliplatin-based chemotherapy.12 A post hoc subgroup analysis of the VELOUR trial (n=533), showed that the expression levels of several plasma biomarkers, like VEGF-A and PIGF, implicated in angiogenesis and correlated with resistance to bevacizumab, was altered after progression on bevacizumab therapy (Table 1).17 In particular, increased expressions of VEGF-A, PIGF, serum amyloid P-component and C-reactive protein have been associated with acquired resistance to bevacizumab,7,8,20 while elevated levels of other biomarkers, like VEGF, T-cadherin and vitamin D binding protein, have been associated with poor prognosis.8,21,22 From these, VEGF-A and PIGF, which have been shown to be involved in the mode of action of aflibercept, demonstrated the most significant change in expression levels (~ 5-fold and 2-fold increase in concentrations, respectively) (Figure 2A).17,23 These results further support the use of aflibercept in the second-line setting for mCRC patients who progressed on bevacizumab. The analysis of the effect of aflibercept treatment on the biomarker patient subgroup of the VELOUR trial revealed that patients with elevated VEGF-A and PIGF baseline levels were associated with improved OS regardless of priorbevacizumab treatment (mOSVEGF-A: 11.9 vs 9.8 months, mOSPIGF: 11.5 vs 10.2 months) (Figure 2B).17,23 Thus, these findings suggest that VEGF-A and PIGF may be implicated in the mechanism of acquired resistance to bevacizumab and that aflibercept represents a viable option for patients who develop resistance to bevacizumab therapy.17
Tissue Biomarkers
In a follow-up retrospective study of the VELOUR trial,24 tumor samples (482 out of 1226 patients met all the sample collection criteria) were used to conduct biomarker analysis, in order to identify patient subgroups, which have a differential response to therapy. Patient subgroups were determined based on certain genetic factors including the mutational status of RAS, KRAS and BRAF genes, and the location of the primary tumor (left-side of the colon vs right-side of the colon). Next-Generation Sequencing (NGS) assay of exon 2 for KRAS, exon 2, 3 and 4 for RAS and V600E for BRAF genes was used to analyze the mutational status, while the tumor location was extracted from the patient’s pathological report.12,24
Genetic Tissue Biomarkers
Based on the NGS data, the patients were divided into the following subgroups (Figure 3A):
-
KRAS exon 2 mutation (MUT)
-
KRAS wild-type (WT)
-
RAS mutation (MUT)
-
RAS wild-type (in the KRAS exon 2 gene or other KRAS- or NRAS genes) (WT)
-
BRAF V600E mutation (MUT)
-
BRAF wild-type (WT)
In this follow-up study, the tissue biomarker subgroups were representative of the intention-to-treat (ITT) population from the VELOUR trial (n=1226) (HR: 0.82 [95% CI: 0.71−0.93]), with respect to the therapeutic effect of aflibercept (HR OS: 0.80 [95% CI: 0.65−0.99]; p=0.043), bevacizumab pretreatment status and overall patient characteristics.24
Based on OS data, there was a trend towards improved efficacy of aflibercept in the RAS wild-type patient subgroup (KRAS: Ratio of HRwild-type mutation [RoHR]: 1.21 [95% CI: 0.79–1.86; p=0.38], RAS: RoHRwild-type mutation: 1.39 [95% CI: 0.90–2.13; p=0.13]).24 Similar results were observed also for bevacizumab and ramucirumab. A trend showing reduced efficacy in the KRAS and RAS mutated patient subgroups was observed in case of these anti-angiogenic agents as well,25,26 which indicates that all three approved anti-angiogenic agents have comparable efficacy in these patient subgroups (Figure 3B).25–27 In case of the BRAF mutant patient subgroup (also RAS wild-type), aflibercept treatment appears to induce a more pronounced survival benefit (RoHRwild-type mutation: 0.49 [0.22‒1.09]; p=0.08).24 In this analysis, the statistical interaction tests were negative for differential response to aflibercept therapy between the wild-type and mutant patient subgroups with respect to OS and PFS. Collectively, none of the mutational subgroups demonstrated a statistically significant differential response to aflibercept treatment in the second line (Figure 3C).
Primary Tumor Location as a Biomarker
About 70% of colorectal cancer patients have a left-sided primary colon tumor. The clinical impact of tumor location (i.e., right- vs left side) was first shown in the phase III E2290 trial. This study demonstrated that patients with right- versus left-sided primary tumors had poorer outcomes (mOS: 10.9 vs 15.8 months, p<0.001).28,29 Developmentally, the left and the right sides of the colon originate from different groups of cells, which renders tumors arising on the two sides to be considerably diverse, both molecularly as well as clinically.30 Several other studies have also confirmed that the location of the tumor has a significant impact on the prognosis of the disease and may also be predictive of response to therapy.30–34 Consistent with this, the addition of cetuximab to chemotherapy in the first-line treatment of mCRC patients prolonged OS and PFS in patients with left-sided primary tumors compared with those having right-sided tumors, first-line treatment with bevacizumab plus chemotherapy provided better outcomes in mCRC patients with right-sided tumors.30,32 Similar results were also observed with ramucirumab in the RAISE study, where ramucirumab led to greater OS benefits in left-sided primary tumors.35 In the case of patients from the VELOUR trial, tumor sidedness was analyzed based on their pathological reports (in 502 patients). Aflibercept efficacy was similar for patients with both left and right-side origin of tumors (HR OS left: 0.86 [0.64 – 0.15], right HR: 0.85 [0.53−1.35], interaction p-value=0.96; HR PFS left: 0.74 [0.46 –1.00], right HR: 0.70 [0.42 – 1.15], interaction p-value=0.69) (Figure 3C).24 A retrospective analysis of 198 mCRC patients from M.D. Anderson Cancer Center showed that right-sided tumors are also associated with other negative prognostic factors like age, consensus molecular subtypes (CMS) 1 and 3, microsatellite instability (MSI), and BRAF mutation status.33 However, more prospective studies are required to confirm that the location of the tumor can be used as a predictive biomarker for the selection of therapy in second-line setting.
CONCLUSIONS
Colorectal cancer is one of the most prevalent cancers worldwide and a large proportion of patients with metastatic disease undergo disease progression. Thus, it is important to plan effective later-line treatment strategies, which aim to prolong survival, manage symptoms and improve quality of life.4 Also, it is essential to identify which patients can potentially benefit from a specific treatment. In this regard, the identification of predictive plasma or tissue biomarkers represents a clinically relevant approach. Analysis of plasma biomarkers of patients who progressed after first-line bevacizumab treatment showed that resistance to this agent is associated with increased plasma levels of VEGF-A and PIGF, which are also targets of aflibercept, indicating that aflibercept could be a good treatment option for the second-line setting irrespective of prior bevacizumab treatment status. The retrospective analysis of the VELOUR trial illustrates that aflibercept is a good candidate for overcoming bevacizumab resistance in mCRC patients. However, future studies including larger patient populations are needed to confirm this effect.
TAKE-HOME MESSAGES
-
Patients with metastatic colorectal cancer undergoing first-line bevacizumab therapy eventually develop disease progression. Other anti-VEGF therapies, such as aflibercept and ramucirumab, may be given to patients as an alternative to bevacizumab.
-
The identification of potential biomarkers to drive the selection of the most appropriate anti-angiogenic drugs is a compelling need. In light of this, new studies are required to prospectively validate the role of predictive angiogenic biomarkers for a rational treatment allocation.
-
High VEGF-A and PlGF serum levels may underlie development of resistance to bevacizumab. Aflibercept targets VEGF-A and PlGF with greater affinity than other anti-angiogenic therapies, and it may provide benefit to patients who have high VEGF-A or PlGF serum levels and may be an effective second-line treatment for those with bevacizumab-induced resistance. Further studies are needed to clarify the importance of these biomarkers in guiding management of patients with colorectal cancer.
Conflict of Interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The author crafted and approved the final manuscript.
_vegf-a_and_pigf_levels_at_baseline_according_to_prior_treatment_with_bevacizumab._b)_me.png)
_genetic_factors_and_the_corresponding_subgroups._b)_aflibercept_has_similar_efficacy_in.png)


_vegf-a_and_pigf_levels_at_baseline_according_to_prior_treatment_with_bevacizumab._b)_me.png)
_genetic_factors_and_the_corresponding_subgroups._b)_aflibercept_has_similar_efficacy_in.png)