BACKGROUND
Approximately 5% of all diagnosed non-small cell lung cancer (NSCLC) patients harbor anaplastic lymphoma kinase (ALK) gene rearrangement, representing a population with specific clinical and pathological features, like no or light smoking history, relatively younger age (median: 52 years) compared with the age for unselected patients with lung cancer (median: 70 years) and adenocarcinoma with signet ring or acinar pathology, which are frequently associated with an aggressive clinical course and a dismal prognosis.1,2 Within the selected patient population of never or light smokers, the incidence of ALK-positive NSCLC has been found to be considerably higher, ranging between 22% (overall) and 33% (without epidermal growth factor receptor [EGFR] mutations) of the patients.3
ALK rearrangements mainly consist of a small intra-chromosomal inversion between the ALK gene and the echinoderm microtubule-associated protein-like 4 (EML4) gene, leading to constitutively active intracellular tyrosine kinase domain of the ALK receptor important for both tumorigenesis and tumor maintenance.4 ALK-positive tumors are highly sensitive to therapy with ALK tyrosine kinase inhibitors (TKIs) which have effectively replaced chemotherapy in the treatment landscape of ALK-positive NSCLC over the last decade. Alectinib is a second-generation ALK TKI indicated as a standard drug for front-line treatment of advanced disease.5–7 Here, the diagnosis and treatment of a 77-year-old male patient with newly diagnosed ALK-positive metastatic NSCLC treated with alectinib as a first-line therapy is presented.
CASE PRESENTATION
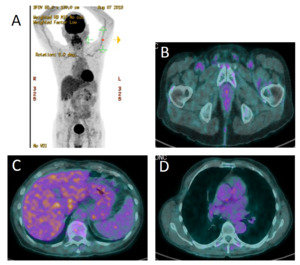
A 77-year-old male patient presented at his general practitioner on 4 May 2018 with worsening dyspnea. The patient was a former smoker who quit smoking at the age of 35, after 10 years of smoking. He had no previous medical problems. At the time of diagnosis, his Eastern Cooperative Oncology Group Performance Score (ECOG PS) was 2 and he presented no comorbidities. On 14 May 2018, computed tomography (CT) was performed, which identified pleural and pericardial effusions with suspicious liver and bone lesions. The patient was referred to the Cardiology Department of the Lausanne University Hospital (CHUV). Pericardial drainage was performed on the same day. Cytological examination of the pericardial fluid confirmed the diagnosis of adenocarcinoma (TTF1+, CK7+). The diagnosis of metastatic lesion of lung adenocarcinoma was further corroborated by liver biopsy. On 18 May 2018, immunohistochemical (IHC) analysis of the liver biopsy specimen showed that tumor cells were ALK-positive. The presence of ALK gene rearrangement was confirmed by fluorescence in situ hybridization (FISH) with a positive cell rate of 100% (analyzed tumor cells, n=50). Except for the pericardial drainage, the patient did not receive any prior treatment. Therapy with 600 mg alectinib twice daily was initiated on 22 May 2018. A magnetic resonance imaging (MRI) performed on 28 May 2018 revealed no brain metastasis. A 18F-choline-positron emission tomography (PET)/CT scan found multiple metastases in lymph nodes, liver, bones and pericardium (Figure 1). The pathological stage was cTxcN3cM1c, stage IVb. The patient continued treatment with alectinib. In September 2019 (about 18 months after treatment initiation), he demonstrated a sustained complete response as confirmed by a PET/CT scan (Figure 2). During the treatment, excellent clinical and biological tolerance was reported, and thus no dose or treatment modifications were required.
LITERATURE OVERVIEW
A better understanding of the pathogenesis of NSCLC has led to the identification of genetic mutations and chromosomal rearrangements such as those in the ALK gene. As a result, multiple drugs acting against these molecular targets have been developed in the last decade, including alectinib, a potent and highly selective inhibitor of ALK. Alectinib received authorization for use in patients with advanced or metastatic ALK-positive NSCLC as either first-line treatment or after progression on a first-generation TKI crizotinib in Switzerland, Europe and the USA.5–7 In addition, alectinib has been included as the preferred front-line and recommended second-line therapy in the current ESMO guidelines (Figure 3).8
The first-line approval was based on the phase III head-to-head ALEX trial, which demonstrated a superior efficacy of alectinib in comparison with crizotinib in 303 patients with ALK-positive NSCLC who did not receive any prior systemic therapy for metastatic disease.10 The results of this study showed that after a median follow-up of approximately 18 months, the median progression-free survival (PFS) was significantly longer with alectinib than with crizotinib (not reached vs 11.1 months), corresponding to a reduction in relative risk of disease progression or death by 53% (p<0.001). Furthermore, the overall response rate (ORR) was higher in the alectinib arm compared with crizotinib arm (82.9% vs 75.5%). Unlike crizotinib, alectinib penetrates into the central nervous system (CNS) due to its lipophilic properties. As a consequence, overall CNS progression was significantly lower among alectinib-treated patients than among crizotinib-treated patients (9.4% vs 41.4%; HR: 0.16; p<0.001).10 An updated analysis of this trial showed that after additional 10 months of follow-up, treatment with alectinib continued to provide an improved median PFS (34.8 months vs 10.9 months; HR for disease progression or death: 0.43).9 Again, in patients with baseline CNS metastases, alectinib was associated with a significantly prolonged median PFS (27.7 months vs 7.4 months). A very similar outcome was demonstrated in another phase III trial (J-ALEX) performed in Asiatic countries, which randomized 207 ALK-positive, crizotinib-naïve NSCLC patients to receive either alectinib or crizotinib.11 At an interim analysis, results showed significantly increased median PFS in the alectinib arm compared with the crizotinib arm (not reached vs 10.2 months), translating into a 66%-reduced risk for disease progression or death (p<0.0001). Finally, the recent phase III ALESIA study which assigned 187 ALK-positive NSCLC patients showed significantly longer investigator-assessed PFS among patients receiving alectinib than among those receiving crizotinib (median PFS, not reached vs 11.1 months; HR: 0.22; p<0.0001).12
These three clinical trials also assessed the safety of alectinib.9,10,12 In general, treatment with this agent was well tolerated, with a better safety profile than crizotinib. The most common adverse events (AE) included nausea, increased alanine transferase (ALT) and diarrhea, which were more prevalent among patients receiving crizotinib; however, myalgia, anemia and increased blood bilirubin were more frequently associated with alectinib than crizotinib.9,10 Of note, despite the prolonged exposure to alectinib (27.0 months vs 10.8 months with crizotinib), the rate of grade ≥3 AE remained lower in this group of patients as shown in the ALEX trial.9 As a result, AEs leading to alectinib dose interruptions and treatment discontinuation were more common in the crizotinib arm.
In the first-line setting, other second- and third-generation ALK TKIs have also been investigated. The phase III ALTA trial compared the clinical benefits of brigatinib, an ALK inhibitor targeting a broad range of ALK mutations, with crizotinib in 275 treatment naïve-patients with advanced ALK-positive NSCLC.13 After the median follow up of 11.0 months and 9.3 months respectively, significantly more brigatinib-treated patients remained progression-free compared with crizotinib-treated patients (67% vs 43%; HR: 0.49 [95% CI: 0.33−0.74]; p<0.001). Furthermore, 71% of the patients receiving brigatinib achieved a confirmed objective response, as compared with 60% of the patients receiving crizotinib. Among patients with brain metastasis, brigatinib was associated with a considerably increased rate of intracranial response (78% vs 29% with crizotinib).
Recently, lorlatinib, a highly potent and brain-penetrant ALK TKI, has shown promising anti-tumor activity in a phase II clinical trial.14 Out of 30 treatment-naïve ALK-positive NSCLC patients, 27 (90%) had an objective response to lorlatinib. Among those with measurable baseline central nervous system (CNS) lesions, intracranial response occurred in 2 out of 3 patients. These results led to an ongoing phase III study comparing the efficacy and safety of lorlatinib with crizotinib at first-line setting in patients with advanced ALK-positive NSCLC.15
CONCLUSIONS
This patient presents a lung adenocarcinoma with ALK-rearrangement, treated with alectinib since May 2018. After 18 months of treatment, the patient continues to demonstrate a good radiological response with excellent clinical tolerability and maintenance of quality of life, which remains an important factor for patients undergoing targeted treatments. Finally, treatment sequencing provides an opportunity for this population of patients; nevertheless, offering access to the best possible treatment with high activity and overall survival benefit, in addition to a good toxicity profile, should be the priority in first-line treatment.
In case of progressive disease, a biopsy should be performed for the identification of the resistance mechanism. Based on the mutational finding, another active TKI, such as crizotinib, brigatinib, lorlatinib or ensartinib might be given. If possible, the patient should be included in a clinical trial which would allow us to better understand this aggressive lung cancer biology.
TAKE-HOME MESSAGES
-
ALK-positive NSCLC is an aggressive disease, frequently associated with liver and/or brain metastases.
-
As this type of tumor is commonly associated with brain metastases, brain MRI should be performed regularly.
-
The best available TKI should be given in first-line treatment.
-
In case of a progressive disease, a re-biopsy should be performed in order to assess the underlying cause of resistance.
Informed Consent
General written consent was obtained from the patient for the publication of this case report and any accompanying images.
COI
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The author crafted and approved the final manuscript.