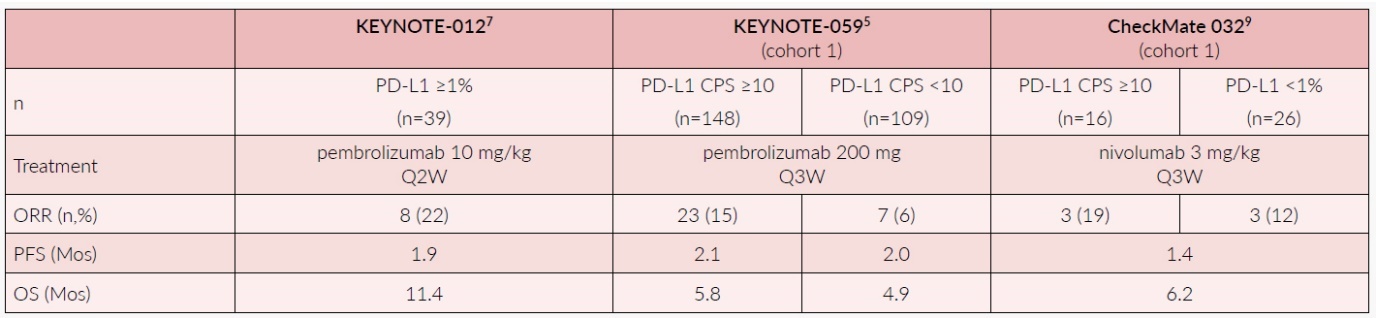
PHASE I AND II TRIALS IN PRE-TREATED POPULATIONS
Phase II trials and phase I expansion cohorts in pre-treated patients demonstrated a better clinical activity of pembrolizumab in PD-L1-positive tumors, with documented overall response rates (ORR) of up to 22%.4–7 Of note, the 17% ORR reported by Kudo et al. (2017) in an unselected esophageal squamous-cell carcinoma (ESCC) cohort of Japanese patients receiving nivolumab after a median of 3 prior therapies was in the same range.8
Tables 1 and 2 summarize the early trials supporting the development of ICIs in esophageal and gastric cancers, respectively.
Checkmate 032 was a large phase I/II trial, enrolling 160 Western patients with advanced esophageal and gastric cancers, progressing after one or more lines of chemotherapy.7 Patients were divided into three different cohorts: nivolumab monotherapy at the standard dose of 3 mg/kg (nivo), nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (nivo1 + ipi3) and nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (nivo3 + ipi1). The primary endpoint of this study was ORR. In general, PD-L1 positivity, detected in 39 out of 127 evaluable patients (31%), was correlated with higher ORR (nivo: 19%, nivo1 + ipi3: 40%, nivo3 + ipi1: 23%), in comparison with PD-L1 negative tumors (nivo: 12%, nivo1 + ipi3: 12%, nivo3 + ipi1: 4%). Interestingly, in the microsatellite instability-high (MSI-H) subgroup (n=11; 8%), the ORR was up to 50%.9
Thus, the combination of nivolumab and ipilimumab is currently under investigation in molecularly selected populations.
KEYNOTE 059 was an open-label phase II trial investigating pembrolizumab in three different cohorts of patients with gastric and gastroesophageal adenocarcinoma: pembrolizumab for patients progressing after two lines of chemotherapy (cohort 1), pembrolizumab in human epidermal growth factor receptor 2 (HER2)-negative patients in first-line, plus cisplatin and a fluoropyrimidine in unselected population (cohort 2), and pembrolizumab alone only in PD-L1-positive (≥1%) patients (cohort 3).10 In cohort 1, the patients with PD-L1-positive tumors (≥1%) obtained an ORR of 15% and this paved the way for the FDA approval of pembrolizumab for refractory gastric and gastroesophageal adenocarcinomas with PDL-1 expression in September 2017.1 So far, this approval is still valid only in the USA but not in Europe or Switzerland.11,12
In cohort 2, pembrolizumab, in combination with standard chemotherapy, was associated with an ORR of 60%, with an encouraging median overall survival (OS) of 13.8 months.8 Even more impressive were the results from cohort 3, in which pembrolizumab monotherapy led to a median OS of 20.2 months in PD-L1-positive patients, although the ORR in this cohort was only 15%.10 These results formed the rationale for larger phase III trials exploring the role of ICIs in the first-line treatment setting for selected patient populations.
PHASE III TRIALS
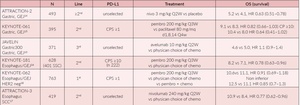
Several phase III trials have analyzed the clinical impact of ICIs in advanced gastroesophageal tumors, in different settings (Table 3).
ICIs in second or later lines
The JAVELIN gastric trial, comparing avelumab with chemotherapy in third-line, did not demonstrate any survival benefits of immunotherapy in the overall patient population.13 In a very similar setting, but in an Asian population, the ATTRACTION 2 trial showed a statistically significant OS benefit with nivolumab in comparison with placebo (5.2 months vs 4.1 months), and the results of this study led to the approval of nivolumab in Japan as a third-line therapy for patients with advanced gastroesophageal cancers.14
In the KEYNOTE-061 study, selected patients with gastric or gastroesophageal junction adenocarcinoma and PD-L1 combined positive score ≥1 (CPS≥1), who progressed after first-line treatment, were randomized to receive either pembrolizumab or paclitaxel.15 No difference was found in the intention-to-treat (ITT) population, whilst in the post-hoc subgroup analysis, the authors observed improved OS in patients with increased PD-L1 CPS ≥10 and treated with pembrolizumab versus chemotherapy (10.4 and 8.0 months, respectively), as well as in patients with MSI-H tumors, irrespective of their PD-L1 status (8.2 months vs not reached, HR: 0.42 [95% CI: 0.13–1.31]).15
PD-L1 expression, used as a biomarker of activity, has also been shown in the KEYNOTE-181 study,16 which demonstrated significantly improved OS with pembrolizumab as the second-line treatment compared to chemotherapy in patients with esophageal adenocarcinoma or squamous cell histology and PD-L1 overexpression. In the ITT population unselected for PD-L1, no differences in OS was detected between the two arms (7.1 months vs 7.1 months), whilst in the squamous cell subgroup, a non-statistically significant benefit was detected (8.2 months vs 7.1 months, HR:0.78 [95% CI: 0.63–0.96]; p=0.0095).16 These findings support the use of pembrolizumab as a new standard for second-line therapy in molecularly selected esophageal and gastroesophageal tumor patients.
The recently published final results of the ATTRACTION-3 trial showed that nivolumab led to a significant survival benefit and better tolerability, compared to second-line chemotherapy, in East Asian population of patients with squamous cell carcinoma of esophagus, progressing after first-line chemotherapy with platinum and fluoropyrimidines.17
Based on these impressive results, this trial set a new standard of treatment in this setting, especially in the Asian population.
Pembrolizumab in first-line
The results of KEYNOTE-062, a randomized study comparing pembrolizumab or pembrolizumab plus chemotherapy with chemotherapy in the first-line setting in patients with PD-L1-positive (CPS≥1), HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma, were presented at 2019 ASCO Annual Meeting.18 The primary endpoint was OS in CPS ≥1 and CPS ≥10 patients. In total, 763 patients, out of which 281 patients had CPS ≥10, were randomized to receive pembrolizumab plus chemotherapy (n=257), pembrolizumab (n=256), or chemotherapy (n=250).
Pembrolizumab, in combination with chemotherapy, did not show superior OS in CPS ≥1 or CPS ≥10 patients, however, a favorable trend was observed. Pembrolizumab was non-inferior to chemotherapy with respect to OS in CPS ≥1 patients as per pre-specified margins. In the highly selected population (CPS ≥10), pembrolizumab versus chemotherapy showed a meaningful benefit in OS (17.4 vs 10.8 months, HR: 0.69). As the trial was not designed for investigating superiority, no statistically relevant conclusions can be drawn from these results. Additionally, as the health-related quality of life (HRQoL) results recently presented at the latest ESMO meeting did not show any relevant differences between the pembrolizumab and chemotherapy arms, it can be concluded that a non-inferiority in terms of OS alone without a better control of symptoms, does not allow us to replace the standard first-line chemotherapy with pembrolizumab, at least for the majority of patients.19
The most exciting results came from the exploratory analysis of 50 patients with MSI-H tumors.20 For the comparison of pembrolizumab versus chemotherapy, the median OS was not reached (95% CI: 10.7–not reached (NR)) versus 8.5 months (95% CI, 5.3–20.8) (HR:0.29; 95% CI: 0.11–0.81). Median OS was not reached (95% CI: 3.6–NR) with pembrolizumab plus chemotherapy compared to 8.5 months (95% CI: 5.3–20.8) with chemotherapy (HR:0.37; 95% CI: 0.14–0.97).20 These results demonstrated that the MSI-H population could benefit from immunotherapy when given in first-line and the patients should be tested upfront as a much better outcome has been observed with an ICI in this randomized phase III trial.
RATIONAL SELECTION OF PATIENTS FOR IMMUNE CHECKPOINT BLOCKADE
Gastroesophageal tumors are usually classified based on their histology, as adenocarcinoma or squamous cell carcinoma. However, a more rational categorization is crucial for the development of novel therapies, such as immunotherapy. With this aim, The Cancer Genome Atlas (TCGA) Research Network proposed a new framework for gastroesophageal cancers, with 4 molecular subtypes in gastric cancers and 3 in esophageal cancers.21,22 Esophageal squamous cell carcinomas resembled squamous carcinomas of head and neck origin more than they resembled esophageal adenocarcinomas, but neither showed any evidence for an etiological role of human papillomavirus.
In gastric cancer, the most immunogenic groups were Epstein-Barr virus (EBV)-positive and MSI-H subtypes. In EBV-positive tumors, a PD-L1/2 overexpression was described, whilst MSI-H was correlated to a high tumor mutational burden.
A proof-of-concept study has been recently conducted in order to elucidate whether these molecular features also correlate with different clinical outcomes.23 This trial included 61 molecularly unselected patients with advanced gastric cancer, treated in second-line with pembrolizumab. The primary endpoint of this phase II study was ORR. An extensive molecular characterization of tissues and circulating tumor (ct) DNA was performed, which showed that MSI-H and EBV positivity, found in 11% and 9% of the trial population, were associated with 85% and 100% ORR, respectively.23 This further supports that EBV positivity and MSI-H represent two strong predictive biomarkers for beneficial treatment with immunotherapy and therefore, patients should always be tested for these biomarkers. On the other hand, the lack of PD-L1 expression was demonstrated to be a strong negative predictive factor (RR: 0% in PD-L1 negative tumors), but only the 50% of the PD-L1-positive patients did respond to ICIs, showing that PD-L1 alone does not represent a reliable biomarker for patient selection, but probably needs to be combined with other molecular and immune signatures for a better patient selection.
CONCLUSIONS
The increasing evidence discussed in this review clearly supports a significant role of immunotherapy in the treatment landscape of gastric and esophageal cancer patients.
So far, the evidence from clinical trial supports the use of immune checkpoint inhibitors (ICIs) in advanced and chemo-refractory treatment settings in patients with PD-L1-positive adenocarcinoma, whilst in case of patients with squamous cell histology, immunotherapy seems to be clinically effective also in unselected populations, especially in Asians.
In first-line, PD-L1 does not play a crucial role in patient selection, as pembrolizumab, in combination with chemotherapy, did not show superior overall survival (OS) in PD-L1 combined positive score (CPS) ≥1 or CPS ≥10 patients, and therefore, it is not a biomarker that can be used in the first-line treatment strategy. Nonetheless, microsatellite instability, as well as Epstein-Barr virus can be used as effective predictive biomarkers and should always be tested upfront. The results of such tests can be used to make crucial treatment decisions, i.e. if positive, pembrolizumab can be used for treatment in first-line.
Future efforts will be aimed at the harmonization of clinical trial results and better baseline molecular assessment to refine the patient selection procedure and to validate novel biomarkers, like high tumor mutational burden, microbiota, immune signatures, for gaining a better understanding of the role of immunotherapy in the treatment of gastroesophageal cancers.
Conflict of Interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The author crafted and approved the final manuscript.
.jpeg)

.jpeg)