BACKGROUND
We present a case of a 64-year-old male patient treated for refractory diffuse large B-cell lymphoma (DLBCL) with involvement of central nervous system (CNS).
DLBCL
DLBCL is the most common neoplasm of the lymphatic system (about 30%), affecting men and women equally.1 The disease has an aggressive natural history and left untreated has median survival of less than 1 year.
DLBCL are a heterogeneous group of neoplasms that develop from lymphoid progenitor cells (common cell of origin). Subtypes can be distinguished via gene expression, genetic aberrations and immunohistochemical markers.2–5
The diagnostic algorithm includes a biopsy, imaging modalities (CT, PET-CT) and bone marrow aspirate. Clinical staging follows the Ann Arbor classification as well as Lugano classification for PET-CT findings.6,7 Prognostication is based on the International Prognostic Index (IPI) or age adjusted IPI.8 Bone marrow involvement, bulky disease and CNS lesions are independent prognostic factors.9,10
Frontline therapy consists in most cases of an immune chemotherapy (usually R-CHOP) with 60–70% cure rates in the case of good response (chemotherapy-sensitive disease). Radiotherapy can be applied upfront or to residual lesions after systemic treatment.11,12 DLBCL of the CNS can occur as a primary manifestation or recurrence and represents an additional adverse prognostic factor. Treatment in the setting of CNS involvement is high-dose methotrexate (MTX).13 In R/R disease (30–40%), therapeutic options include salvage chemotherapy (usually R-ICE or R-DHAP) followed by high dose chemotherapy (usually BEAM) with autologous stem cell rescue (ASCT).14,15 In case of chemotherapy-refractory disease, there was previously no curative therapy available. Just recently, novel immune cell therapies, so-called chimeric antigen receptor (CAR) T cells have become available for this patient population. This is the first therapy that can provide a cure to DLBCL that cannot be controlled by chemo- and other conventional immunotherapies.
CAR T cells
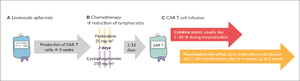
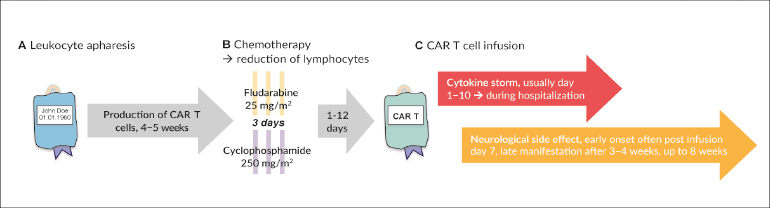
CAR T cells are autologous lymphocytes that are genetically modified to be specifically targeted against CD19-positive cells. While antigen recognition of natural T cells is based on the presentation of the corresponding antigen by MHC molecules, tumor cells often show a loss of MHC expression (Figure 1).16 The chimeric antigen receptor recognizes unprocessed protein antigens without the need for MHC molecules. The constructs consist of the extracellular, antigen-specific single-chain variable fragment of a monoclonal antibody linked to intracellular co-stimulatory signaling domains.17 The production of CAR T cells is a complex process that involves several steps and hence is time-consuming (about 4 weeks). Before infusion of the product patients undergo lymphodepleting chemotherapy (usually with cyclophosphamide and fludarabine). The only regulatorily approved and commercially available products are targeted against CD19 (tisagenlecleucel and axicabtagene ciloleucel) for treatment of hematologic malignancies (R/R B-ALL and DLBCL).18–21
Of major clinical concern is the massive immune reaction caused by strong cytokine release (cytokine release syndrome, CRS) in response to CAR T cell infusion as well as life-threatening neurotoxicity (up to 20% of cases).22 Usually, symptom control can be achieved with supportive measures but might require the administration of tocilizumab or corticosteroids.
CASE PRESENTATION
Treatment history
The patient was diagnosed with DLBCL in November 2017. Initially, the lymphoma manifested with extensive involvement of the facial skull, especially in the area of the orbital cavity. First-line therapy consisted of R-CHOP and CNS prophylaxis using high-dose MTX. MTX was discontinued after 1 cycle due to transient renal insufficiency. By March 2018, the patient achieved complete remission, albeit of short duration. The first relapse occurred in May 2018 with extensive soft tissue and lymph node involvement, as well as CNS involvement. Salvage therapy was carried out in analogy to the MARIETTA protocol with 3 cycles of MATRIX and 2 cycles of R-ICE, followed by high-dose chemotherapy with carmustine and thiotepa and autologous stem cell transplantation (ASCT) in September 2018. Unfortunately, the first follow-up PET-CT imaging at 3 months post-ASCT revealed new metabolic disease activity, now as subcutaneous lesions in the skin of the left lower leg. This second relapse initially manifested with localized lesions without systemic or CNS manifestations (according to imaging PET-CT and cranial MRI). Therefore, radiation to the lesion was applied. However, during radiation multiple additional subcutaneous lymphoma nodules appeared in area of the hip, thighs, arms and chest wall, with further progression on repeated examination. In the face of heavily pretreated progressive DLBCL, the consensus of our multidisciplinary tumor board found CAR T therapy to be the most promising option. The indication for CAR T cell treatment was fulfilled according to its approved registration in Switzerland, but coverage of costs was unclear.
We submitted our request and all medical records to the insurance company. After 46 days of negotiation, a positive response for coverage and reimbursement of the treatment by insurance and cantonal authorities was obtained. During this time, the patient urgently required therapy, as new bulky lesions in the muscles of the lower extremity appeared that were both debilitating and painful (Figure 3A). We immediately carried out the lymphapharesis since further treatments could potentially deplete T cells and suppress and impair their biological function, and then proceeded to bridging therapy. In a single leukapheresis a total of 11.34 x 109 CD3-positive T cells was harvested (target value: ≥ 1 x 109 ) and cryopreserved in liquid nitrogen. The patient was put back on chemotherapy, but neither responded to another cycle of R-ICR nor to ibrutinib, or to R-DHAOx. Table 1 summarizes the treatment timeline prior to CAR T therapy.
As soon as reimbursement issues were clarified, the order for tisagenlecleucel was placed, a production slot reserved and the cells were shipped to Morris Plains, New Jersey, US.
Due to the above-mentioned potential risks and complications after CAR T cell therapy (CRS, neurotoxicity), a pre-CAR T check-up similar to hematopoietic stem cell transplantations was recommended. This check-up involves staging of the lymphoma (if indicated including CNS imaging and lumbar puncture, and electroencephalography), echocardiogram, pulmonary function testing, infectious disease serologies and blood work.
PET-CT and cranial MRI imaging prior to CAR T cell infusion revealed progressive disease of muscular and subcutaneous lymphoma manifestations but also showed a new lesion in the right frontal area of the dura, which appeared to be outside the CNS parenchyma. Yet, in the cerebrospinal fluid, 5 cells/µl were detected that were immunophenotypically clonal B cells. Patients with CNS involvement were excluded from all published clinical trials. Experienced colleagues in the US report on the use of CAR T cells in patients with a history of CNS lymphoma involvement; however, they do not administer CAR T cells in patients with an active CNS disease. After long discussions in our group, with colleagues in the US (University of Pennsylvania) and the patient and his wife regarding the benefit and hazard of this therapy, we decided to carry out the CAR T cell therapy.
CAR T therapy
As preparation for CAR T cell infusion, the patient received 3 days of lymphodepleting chemotherapy consisting of fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2), which he tolerated without significant complications. This lymphodepletion serves to improve the homeostatic expansion of the CAR T cells once infused into the lymphopenic environment.
On day 2 post-chemotherapy, 21 ml cell suspension were thawed at the patient’s bedside and infused, containing a total of 1.86 x 106 CAR-positive viable T cells.
For the next 10 days, the patient was closely monitored for CRS and neurologic toxicity. From day 2 on, remitting fevers occurred despite continuous antipyretic measures, accompanied by chills and fatigue corresponding to CRS grade 1. Low blood pressures were measured (below 90 mmHg systolic) but normalized with boli of fluids (CRS grade 2). The patient was at no time hemodynamically unstable, nor did he require oxygen. Due to the fevers and a significant increase of C-reactive protein, empirical antibiotic therapy was initiated with piperacillin-tazobactam – even though an infectious cause of the inflammation was less likely. Figure 2 displays the time course of clinical and laboratory parameters and illustrates the dynamics with peak and subsequent resolution of CRS within approximately 10 days after CAR T cell infusion. In the course of his hospitalization, the patient exhibited no further CRS-related symptoms.
Of special note, clear neurological symptoms were absent; however, fine motor skills were clearly impaired. The severe fatigue may also be interpreted as a sign of neurotoxicity.
On clinical examination, a cutaneous lymphoma manifestation on the thorax wall seemed initially progressive, assessed via palpation. In addition, new lesions and swelling the left lower leg occurred that were suspicious for new lymphoma manifestations. A biopsy, however, did not confirm lymphoma infiltration in the new nodules, and the large chest-wall lesion was rapidly decreasing in size after about 8–10 days. With swelling of the leg, duplex ultrasonography was done to rule out thrombosis. In fact, this examination revealed a fluid collection in the area of prior-lymphoma-manifestation, which was interpreted as decaying tissue.
In Switzerland and in most European countries, CAR T cell treatments are typically done in an in-patient setting with hospitalization until day 10, as daily examinations are mandatory until day 10 after CAR T cell infusion. The patient presented here, however, required an extended hospitalization due to excessive fatigue and weakness. When he left the hospital, he continued to have severe cytopenias that lasted over several weeks. Prolonged cytopenias that are typically less severe but last considerably longer than those following standard chemotherapies are commonly observed during CAR T cell treatments and are most likely due to the chronic inflammation induced by the immunotherapy.23 Upon discharge from our institution, the lymphoma lesion in the area of the chest wall was completely resolved, the painful tension of the muscles of the left leg had disappeared, and the patient was in good clinical condition.
Follow-up examination by PET-CT revealed a partial remission of the muscular lymphoma bulks at 5 weeks post-CAR T infusion, that further improved, but was still weakly detectable at 10 weeks. Of note, PET-CT cannot distinguish the metabolic activity of lymphoma cells from an ongoing immune response. Cranial MRI revealed complete remission of the dural lesion at 10 weeks post-CAR T cell infusion (Figure 3).
DISCUSSION
Refractory or relapsed DLBCL is a difficult-to-treat disease entity with a poor prognosis under prior standard of care therapeutic regimen. The development of tumor-specific CAR T cells is beginning to revolutionize the field of hemato-oncology and has shown promising results in R/R DLBCL. At our center, we have treated 10 patients to date. While long-term follow-up data are not available, initial response assessments are in line with the published study results.
The patient presented here had progressive refractory DLBCL after 9 lines of conventional therapy with main manifestations in the skin and lymph nodes. Additionally, he presented with lymphoma manifestations within the cranium, where active CNS involvement could not be completely ruled out at the time of planned CAR T therapy. The results reported demonstrate a very good partial response at week 10 post-treatment and complete resolution of CNS lesions, with minor side effects in this heavily pretreated patient. Our results are in concordance with a recent report by Frigault et al. in patients with secondary CNS lymphoma that demonstrated only minor toxicity despite evidence for CAR T cell activity.24
Conclusions
We present a patient that illustrates a challenging clinical scenario, the availability of a revolutionary treatment option, the need for unconventional treatment decisions, mild side effects, and an excellent response. CAR T cell therapy offers a realistic chance of cure to this heavily pretreated patient population that until recently would have had no hope of cure. Even though follow-ups are short and long-term results are missing the available experiences and studies are outstanding. Given our and the experience of others, CAR T cell therapy for DLBCL patients with difficult-to-treat disease could become the new standard of care and should not be withheld from this patient cohort.
Patient consent
Informed consent has been obtained from the patient.
The submission of this case report has been supported by Novartis, while Novartis has had no influence over the content. Also, the authors did not receive any honoraria for writing this case report.
Conflict of Interests
Dr Antonia Maria Müller has participated in advisory boards by Novartis, KITE/Gilead and Celgene. Dr Alexander Ring has no conflicts of interest.
Author Contributions
All authors contributed to and approved the final manuscript.