BACKGROUND
Prostate carcinoma is one of the most common cancers in men in industrialized nations, ranked third after lung and colon cancer with regard to cancer-related mortality.1 Around 10% of the men diagnosed with prostate carcinoma demonstrate other metastases, which are usually successfully treated with androgen deprivation therapy (ADT).1 However, this therapy response is not lasting, and nearly all tumors achieve a metastatic castration-resistant status sooner or later.1 There are several different options available for the treatment of metastatic castration-resistant prostate carcinoma (mCRPC). Here, we present the diagnosis and treatment of a patient with metastatic prostate carcinoma receiving anti-hormonal therapy who had an increasing protein-specific antigen (PSA) value, increasing pain in the lumbar region and hypesthesia in the region of the groin and proximal upper thigh. Finally, a literature overview of systemic therapies for mCRPC is also provided.
CASE PRESENTATION
The 78-year-old patient was initially presented in January 2016 with a steadily increasing PSA value while receiving ADT with goserelin acetate (10.8 mg/every 3 months). The main symptoms included increasing lumbar pain, hypesthesia in the L1 region on the left side (groin, proximal upper thigh), and increasing PSA value (doubling time approx. 2 months).
In January 2012, the patient was diagnosed with prostate carcinoma (Gleason 4 + 5 = 9, T1b, cN1, M0). His general condition was excellent, and he had no comorbidities. An MRI of the prostate showed an enlargement up to the mesorectal fascia, a pronounced growth beyond the capsule with uneven outgrowths laterally to the right, infiltration of the seminal vesicle and neurovascular bundle, a small area with a capsular transgression dorsally to the left and a suspicious iliacal external lymph node on the left side. The scintigraphy gave no indication of skeletal metastases. The PSA value was initially 7.8 µg/L. Anti-androgen therapy was initiated with goserelin acetate in January 2012. Transurethral resection of the prostate in February 2012 returned a normal finding for the prostatic urethra. Based on the patient’s preference, radiotherapy was initiated. The PSA value fell to 0 µg/L in the course of the therapy. The patient tolerated continuous ADT with goserelin acetate well, until the appearance of the first symptoms at the end of 2015.
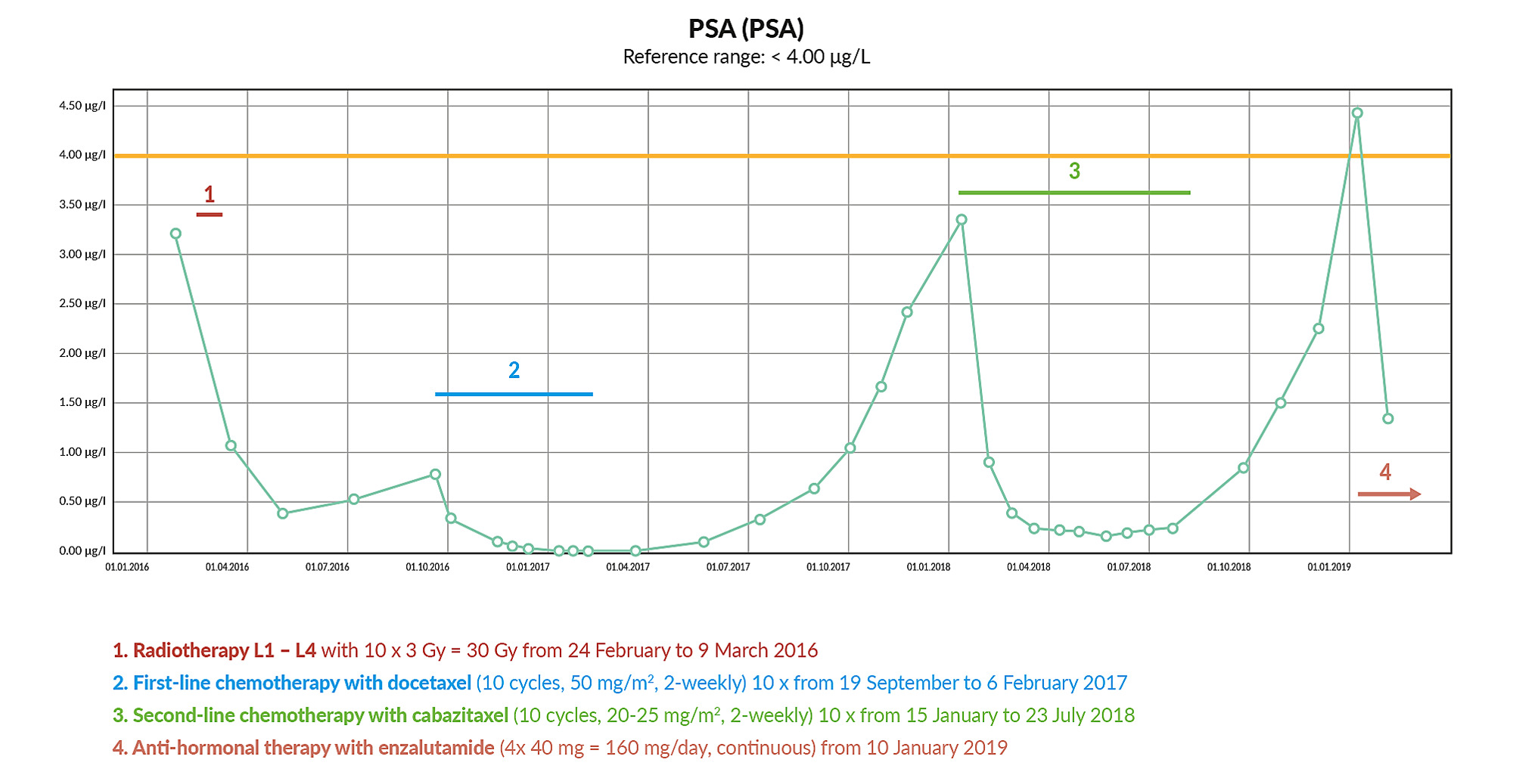
In January 2016, the patient was referred by the treating urologist after the PSA value had risen to 3.21 µg/L. 18F-choline-PET/CT demonstrated multiple lymph nodes and bone metastases (Figure 1). The patient was diagnosed to have metastatic castration-resistant prostatic carcinoma (M1 mCRPC). In February and March 2016, analgesic radiotherapy was performed in the L1-L4 region. In May 2016, this therapy led to a substantial reduction in the PSA value (0.40 µg/L), followed by stable progression with a value of 0.53 µg/L in July 2016 (Figure 2). The patient was asymptomatic at this time. A SPECT-CT in August 2016 showed a progressive skeletal metastization with new metastases in the third ribs, spinous processes of T1 and T12, progressive metastasis in L5 and residual metastization in L1 and L4. Pulmonary metastization and a partial, low-grade regressive lymph node metastization were also determined.
Chemotherapy was performed with docetaxel (10 cycles, 50 mg/m2, 2-weekly) from September 2016 to February 2017, which led to the reduction of PSA value by > 90% (Figure 2). After 8 cycles, a SPECT-CT in January 2017 showed a response of all lesions with regression in the size of the pulmonary metastases and reduced technetium uptake by the osseous lesions. Adverse reactions included nail changes (panaritium), secondary infections, water retention and peripheral neuropathy. In September 2017, a choline-PET/CT showed newly occurring urethral metastasis, weakly choline-positive lymph node metastasis (common iliac, left side), minimal choline uptake by the lumbar metastases, choline-positive metastases in the sacrum, L5 of the seventh rib on the right side and in T11. A further choline-PET/CT in January 2018 showed an overall progression of the findings with a morphological and metabolic increase of the lymph node metastases (common iliac, left side), progressive osseous metastases and newly occurring bone metastases in the coccyx and T2. There were also newly suspected metabolically active mediastinal and hilar lymph node metastases on the left side, multiple, partly minimally growing pulmonary nodules bilaterally, as well as a metabolically minimally progressive mass in the distal ureter.
Between January and July 2018, second-line chemotherapy was performed with cabazitaxel (10 cycles, 20-25 mg/m2, every 2 weeks). During this therapy, the PSA value fell by > 90% from 3.35 µg/L to 0.25 µg/L (Figure 2). Regression was also seen in all lesions. Exceptions to this were a morphologically and metabolically stationary thickening in the distal urethra on the left side and mediastinal and hilar lymph node metastases which remained the same size imaged in a choline-CET/PT in August 2018. The therapy was excellently tolerated with the exception of a mild accentuation of the polyneuropathy. The PSA level increased again after the end of the therapy, up to a value of nearly 4.5 µg/L in January 2019 (Figure 2). In addition, the patient suffered again from increasing lumbovertebral pain with paraesthesia in the L4/5 region on the left side.
In January 2019, anti-hormonal therapy with enzalutamide (4 x 40 mg = 160 mg/day, continuous) was initiated, under which the PSA value substantially reduced within the first 4 to 12 weeks (Figure 2).
LITERATURE OVERVIEW
One treatment option for mCRPC patients is systemic chemotherapy with taxanes, docetaxel or cabazitaxel.2,3 Docetaxel acts on the cell’s microtubule system and thus inhibits tumors.2 In the phase III TAX-327 study, which enrolled a total of 1,006 patients with mCRPC, the median survival time was significantly prolonged under first-line therapy with docetaxel compared to mitoxantrone (18.9 vs. 16.5 months, respectively; p=0.009).4 Based on these data, docetaxel in combination with prednisone/prednisolone is indicated for the treatment of patients with mCRPC.2 Cabazitaxel, like docetaxel, is an anti-neoplastic active substance which acts on the cell’s microtubule system.3 For patients with mCRPC, who are progressive under docetaxel, resistance can be overcome effectively by switching to cabazitaxel. In the phase III TROPIC study, 755 patients with mCRPC had previous disease progression under or after a regimen containing docetaxel.5 In the final analysis, patients in the post-docetaxel setting survived significantly longer under cabazitaxel than under mitoxantrone (median of 15.1 months vs. 12.7 months, respectively, p<0.0001).5 Cabazitaxel - in combination with prednisone/prednisolone - is thus indicated for the second-line therapy of patients with mCRPC who have received previous treatment with docetaxel.3
Apart from chemotherapy, there is the option of providing mCRPC patients with anti-hormonal treatment with abiraterone acetate or enzalutamide.6,7 Abiraterone acetate is transformed in vivo to abiraterone, which selectively blocks the enzyme CYP17, thus inhibiting androgen biosynthesis in the testicles, adrenal glands and tumor tissues.6 In the phase III COU-301 study, which enrolled a total of 1,195 patients with mCRPC who had previously been treated with docetaxel, median overall survival was significantly prolonged under abiraterone/prednisone compared to therapy with prednisone alone (15.8 vs. 11.2 months; p<0.0001).8 In the phase III COU-AA-302 study, 1,088 chemotherapy-naive, asymptomatic and/or mildly symptomatic mCRPC patients without visceral metastases received abiraterone or placebo (each with prednisone/prednisolone).9 In the final analysis, a prolongation of median overall survival by 4.4 months was also achieved for these patients receiving abiraterone relative to a comparison therapy (34.7 vs. 30.3 months, respectively; p=0.0033).9 Thus abiraterone, in combination with prednisone/prednisolone and LHRH agonists, is indicated as a second-line therapy for patients with mCRPC after previous treatment with docetaxel or as first-line therapy for asymptomatic/mildly symptomatic patients without visceral or liver metastases.6
Enzalutamide is an inhibitor of the androgen receptor pathway, suppressing the binding of androgens to the androgen receptor as well as the downstream steps in the signaling cascade.7 A total of 1,199 mCRPC patients previously treated with docetaxel participated in the phase III AFFIRM study.10 In these patients, enzalutamide prolonged median overall survival by 4.8 months compared to the placebo group (18.4 vs. 13.6 months, respectively; p<0.001).10 The phase III PREVAIL study enrolled 1,717 asymptomatic or mildly symptomatic mCRPC patients who were chemotherapy-naive.11 Enzalutamide prolonged survival in this patient group by 4 months, from 35.3 months to 31.3 months.12 The proportion of patients with radiologically progression-free survival after 12 months was 65% under enzalutamide and 14% in the placebo group; this corresponded to an 81% risk reduction (p<0.001).11 Enzalutamide, in combination with LHRH agonists, is approved on this basis for the treatment of mCRPC patients with asymptomatic/mildly symptomatic progression after androgen therapy failure and for progression under or after docetaxel therapy.7
Patients with mCRPC also have the option of radiation therapy, e.g., with the intravenously injectable alpha radiation-emitting radium 223.13 The ALSYMPCA pivotal study enrolled a total of 921 mCRPC patients with symptomatic, osseous, but not visceral metastases.14 In the final analysis, those patients, who were treated with radium 223 in addition to the best possible standard therapy, survived 3.6 months longer than those who received a placebo instead of the radionuclide (14.9 vs. 11.3 months, respectively; p<0.001).14 The first skeletal complications also occurred with a delay (15.6 vs. 9.8 months; p<0.001).14 Radium 223 is thus indicated for the treatment of CRPC patients with symptomatic bone metastases and without known visceral metastases. However, the European Medicines Agency (EMA) recommends certain restrictions regarding the use of radium 223. According to EMA, only patients who have received at least two previous metastatic prostate cancer treatments (with bone metastasis) and patients who are ineligible for other treatments can be treated with radium 223.13,15
Conclusion
Radiotherapy was associated with a PSA response and was able to prolong systemic therapy. Subsequent chemotherapies with docetaxel and cabazitaxel led to a fall of the PSA value by >90%; this is a positive outcome. After the taxane treatments, the patient had a long therapy-free interval of nearly a year (docetaxel) or 6 months (cabazitaxel). The response to enzalutamide cannot yet be evaluated conclusively. However, multiple studies show that the fall of the PSA value within the first 4 to 12 weeks - as observed in this case - is very important.16 The adherence of the patient was excellent to that point, with the therapies being primarily administered intravenously. Despite adverse reactions, therapy was not discontinued prematurely.
Considered retrospectively, the therapy decision was correct. However, there are several other treatment pathways for mCRPC, which would have been possible. The therapy sequence should be independent of age and tumor-specific risk factors. With regard to the imaging, new procedures such as choline- or PSMA-PET/CT should also be considered. These open up the possibility of using salvage options. Regular imaging is, therefore, also very important as the PSA value alone is not always informative.
Informed Consent
General written consent was obtained from the patient for the publication of this case report and any accompanying images.
COI
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The author crafted and approved the final manuscript.
_using_18f-choline-pet_ct.jpeg)

_using_18f-choline-pet_ct.jpeg)