Introduction
Radioligand therapy (RLT) represents a rapidly evolving modality in precision oncology, combining targeted molecular delivery with cytotoxic radiation to selectively eradicate malignant cells while sparing surrounding healthy tissues. In prostate cancer, prostate-specific membrane antigen (PSMA)-directed RLT using Lutetium-177 [177Lu]Lu-PSMA-617 has demonstrated substantial clinical benefit across disease stages, establishing a foundation for expanding indications and novel combination strategies.1,2 Beyond prostate malignancies, RLT also plays an important role in the management of other solid tumors, including peptide receptor radionuclide therapy (PRRT) with [177Lu]Lu-DOTATATE in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs).3–5 The field is further expanding toward α-emitter-based therapies, optimized ligand design and next-generation agents targeting alternative receptors on tumor cells. Parallel advances in imaging, dosimetry and molecular characterization have enabled a more individualized approach, linking diagnostic tracer uptake with therapeutic efficacy. Combination regimens integrating RLT with systemic, hormonal and immune therapies are also under active investigation, aiming to extend its reach beyond late-stage disease into neoadjuvant and adjuvant settings. This review article highlights a selection of recent advances in RLT presented at the 2025 European Society for Medical Oncology (ESMO) Annual Congress, including data from phase III clinical trials, as well as early phase studies of emerging agents, novel targets and innovative therapeutic strategies in prostate cancer.
212Pb-DOTAMTATE for therapy of gastroenteropancreatic neuroendocrine tumors
RLT using [177Lu]Lu-DOTATATE is a recommended second-line treatment after somatostatin analogs (SSAs) for patients with somatostatin receptor (SSTR)-positive GEP-NETs.6,7 In progressive disease, few effective treatment options remain. Emerging evidence suggests that targeted α-particle therapy may overcome resistance to β-emitters such as 177Lu or represent a promising alternative.8 In contrast to β-emitters, α-emitters produce highly energetic, short-range radiation, classified as high linear energy transfer, and predominantly cause irreparable DNA double-strand breaks, thus allowing for potentially more efficient and targeted tumor cell killing.8,9 Lead-212 [212Pb]Pb-DOTAMTATE is a next-generation RLT utilizing α-emission, with a recommended phase II dose regimen of four doses at 2.50 MBq/kg (67.6 μCi/kg).10 Recently, data from the phase II ALPHAMEDIX-02 trial were presented, which evaluated [212Pb]Pb-DOTAMTATE in patients with SSTR-expressing unresectable or metastatic GEP-NETs.11,12
The study included two cohorts: RLT-naïve patients (cohort 1, n=35) and patients previously exposed to RLT who had progressive disease after receiving ≤4 doses of [177Lu]Lu-SSA and received their last dose ≥6 months before study treatment initiation (cohort 2, n=26).11,12 Patients received [212Pb]Pb-DOTAMTATE at 2.50 MBq/kg every eight weeks for up to four cycles. The primary endpoints were the objective response rate (ORR) and incidence of adverse events (AEs). Progression-free survival (PFS) and overall survival (OS) were among the key secondary objectives.
In RLT-naïve patients, previously reported data showed that [212Pb]Pb-DOTAMTATE achieved an ORR of 55.6% and a median duration of response of 17 months.13 The 24-month PFS and OS rates were 74.3% and 90.7%, respectively. Overall, [212Pb]Pb-DOTAMTATE exhibited a manageable safety profile.
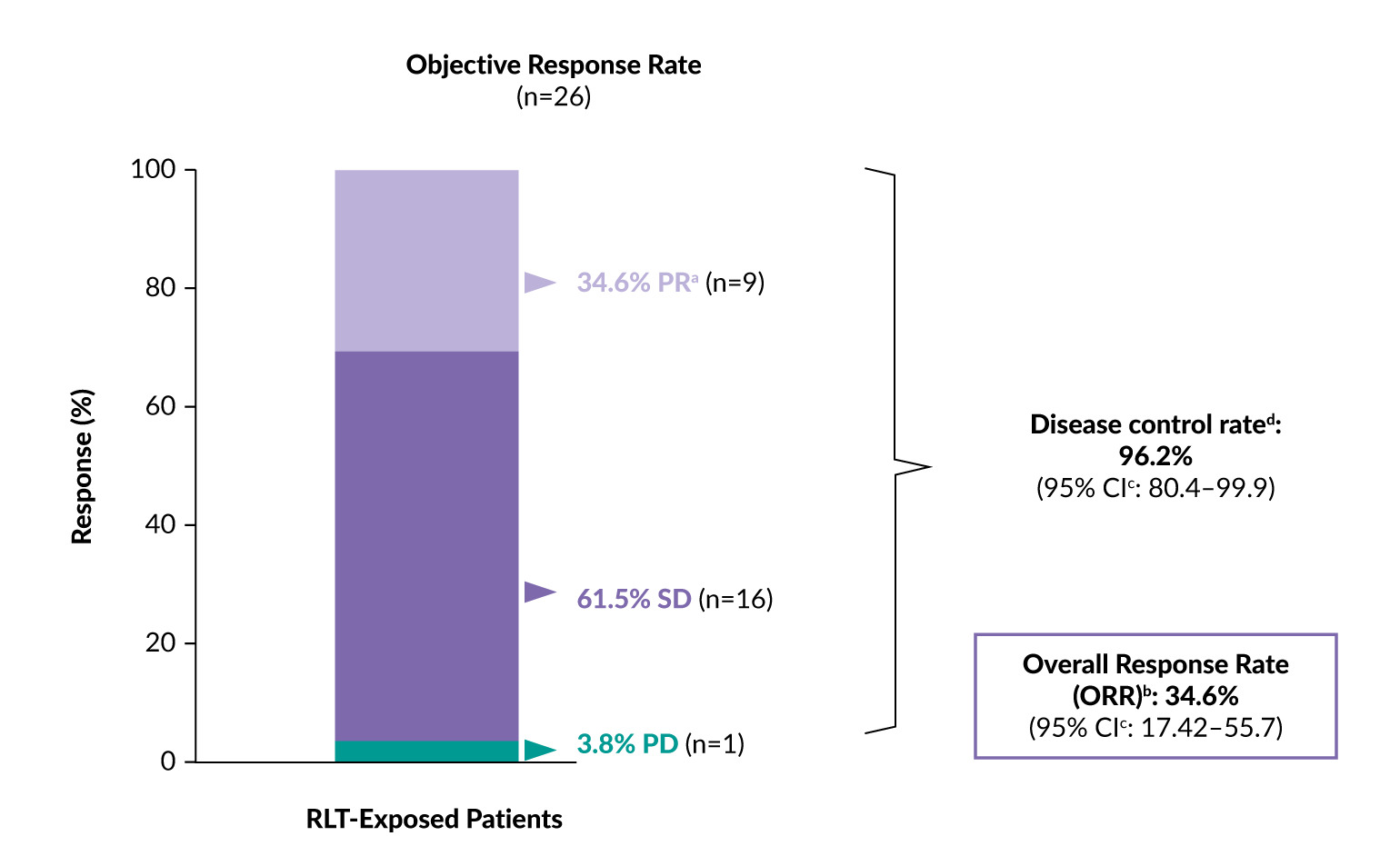
In the RLT-exposed cohort, [212Pb]Pb-DOTAMTATE resulted in a clinically meaningful ORR of 34.6% and a disease control rate of 96.2% at a median follow-up of 14.6 months (Figure 1).12 All nine patients who achieved partial remission demonstrated durable responses maintained for ≥18 months. Only one patient had progressive disease, whereas 16 (61.5%) had stable disease. Importantly, cases deemed stable on conventional imaging can show a response when evaluated by positron emission tomography/computed tomography (PET/CT) of SSTRs such as [68Ga]Ga-DOTATATE.14 At 18 months, the PFS rate was 82.6% and the OS rate was 85.1%.
Nearly 60% of patients with previously untreated metastatic triple-negative breast cancer (mTNBC) are ineligible for immunotherapy targeting programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1). Among those who receive first-line treatment for mTNBC, approximately 50% do not proceed to second-line therapy and quality of life (QoL) deteriorates substantially with each subsequent line of treatment, highlighting the urgent need to deliver the most effective therapy as early as possible while preserving QoL.
[212Pb]Pb-DOTAMTATE demonstrated an acceptable safety profile, allowing most patients (84.6%) to complete all four doses of treatment.12 Over half of the treatment-emergent adverse events (TEAEs) were grade 1–2 (57.5%), with alopecia (84.6%), fatigue (73.1%) and nausea (69.2%) being among the most common. AEs of special interest included dysphagia (any grade, 57.7%; grade ≥3, 15.4%) and renal events (any grade, 7.7%; grade ≥3, 7.7%), with no cases of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) observed.
In summary, [212Pb]Pb-DOTAMTATE showed impressive efficacy and a favorable benefit/risk profile in RLT-exposed patients with advanced GEP-NETs.12 Interestingly, the occurrence of dysphagia suggests treatment effects on the esophagus, where the uptake of [68Ga]Ga-DOTATATE is uncommon. Finally, its short half-life of 10.6 hours (compared with 6.7 days for 177Lu) may pose logistical challenges, yet it offers potential advantages in terms of patient social distancing precautions and disposal concerns.10,15
[177Lu]Lu-PSMA-617 versus docetaxel after ARPI in mCRPC
In metastatic castration-resistant prostate cancer (mCRPC), treatment sequencing following progression on androgen deprivation therapy (ADT) and androgen receptor pathway inhibitors (ARPIs) represents an important clinical challenge. Docetaxel is a recommended treatment option for chemotherapy-naïve patients, with recent data showing a median radiographic PFS (rPFS) of 8.3 months and OS of 19.0 months.16,17 Comparable benefit has been observed in taxane-naïve patients with PSMA-positive disease treated with [177Lu]Lu-PSMA-617 (median rPFS, 9.30 months; median OS, 24.5 months) in the phase III PSMAfore trial.18,19 In this study, [177Lu]Lu-PSMA-617 demonstrated significant improvement in rPFS (HR: 0.41 [95% CI: 0.29–0.56]; p<0.0001) but not in OS (HR: 0.91; p=0.20; crossover-adjusted, HR: 0.59) compared with a change in ARPI. The investigator-initiated phase II CCTG PR.21 trial directly compared the two treatment options to address this unresolved question.20
The trial enrolled patients with PSMA PET-positive mCRPC who had progressed on ARPI therapy and had not received chemotherapy for castration-resistant disease.20 A total of 199 patients were randomized 1:1 to receive either [177Lu]Lu-PSMA-617 (7.4 GBq every six weeks [Q6W] for six cycles) or docetaxel (75 mg/m2 Q3W for 12 cycles) with cross-over at progression. The primary endpoint was rPFS, with OS among the secondary endpoints.
The trial failed to demonstrate a benefit with [177Lu]Lu-PSMA-617 over docetaxel, with a median rPFS of 8.6 months versus 10.7 months, respectively (HR: 1.01 [90% CI: 0.77–1.31]; one-sided p=0.51).20 Interestingly, the mean standardized uptake value (SUVmean), previously reported to be predictive of PSMA-RLT efficacy,21 was found to be prognostic but not predictive in this study.20 rPFS was longer in patients with high SUVmean compared with those with low SUVmean, irrespective of treatment (docetaxel, HR: 0.44 [90% CI: 0.29–0.65]; two-sided p=0.0004; [177Lu]Lu-PSMA-617, HR: 0.66 [90% CI: 0.45–0.98]; two-sided p=0.079).
OS favored docetaxel over [177Lu]Lu-PSMA-617 as the initial treatment (HR: 1.64 [95% CI: 1.14–2.35]; two-sided p=0.02), with a median OS of 18.2 months versus 14.3 months. Notably, 56 patients crossed over to [177Lu]Lu-PSMA-617 upon progression on docetaxel compared with 38 in the RLT arm, a discrepancy that requires further examination to ensure accurate interpretation of the study findings.
Importantly, [177Lu]Lu-PSMA-617 had a more favorable safety profile than docetaxel, with fewer grade 3–4 treatment-related AEs (TRAEs) (13% vs 34%), as well as treatment discontinuations due to TRAEs (one patient vs 15 patients).
Together, these data demonstrate that while [177Lu]Lu-PSMA-617 was significantly better tolerated, there was no rPFS benefit over docetaxel in patients with mCRPC after ARPI.
[177Lu]Lu-PNT2002 as neoadjuvant treatment to SBRT in recurrent oligometastatic HSPC
Stereotactic body radiotherapy (SBRT) has demonstrated favorable clinical outcomes in patients with oligometastatic prostate cancer, with most evidence derived from retrospective studies and two randomized phase II trials (STOMP, ORIOLE).16,22–24 The phase II LUNAR trial evaluated the value of adding PSMA-targeted therapy with [177Lu]Lu-PNT2002 RLT to salvage SBRT in this setting.25
The trial enrolled patients with oligorecurrent hormone-sensitive prostate cancer (HSPC) by PSMA PET/CT (1–5 lesions) who had not received ADT within six months.25 A total of 92 patients were randomized 1:1 to receive either [177Lu]Lu-PNT2002 (two cycles of 6.8 GBq, eight weeks apart) prior to SBRT or SBRT alone. The primary endpoint was PFS. Forty-five patients received [177Lu]Lu-PNT2002 plus SBRT and 42 received SBRT alone, as five patients withdrew consent upon randomization.
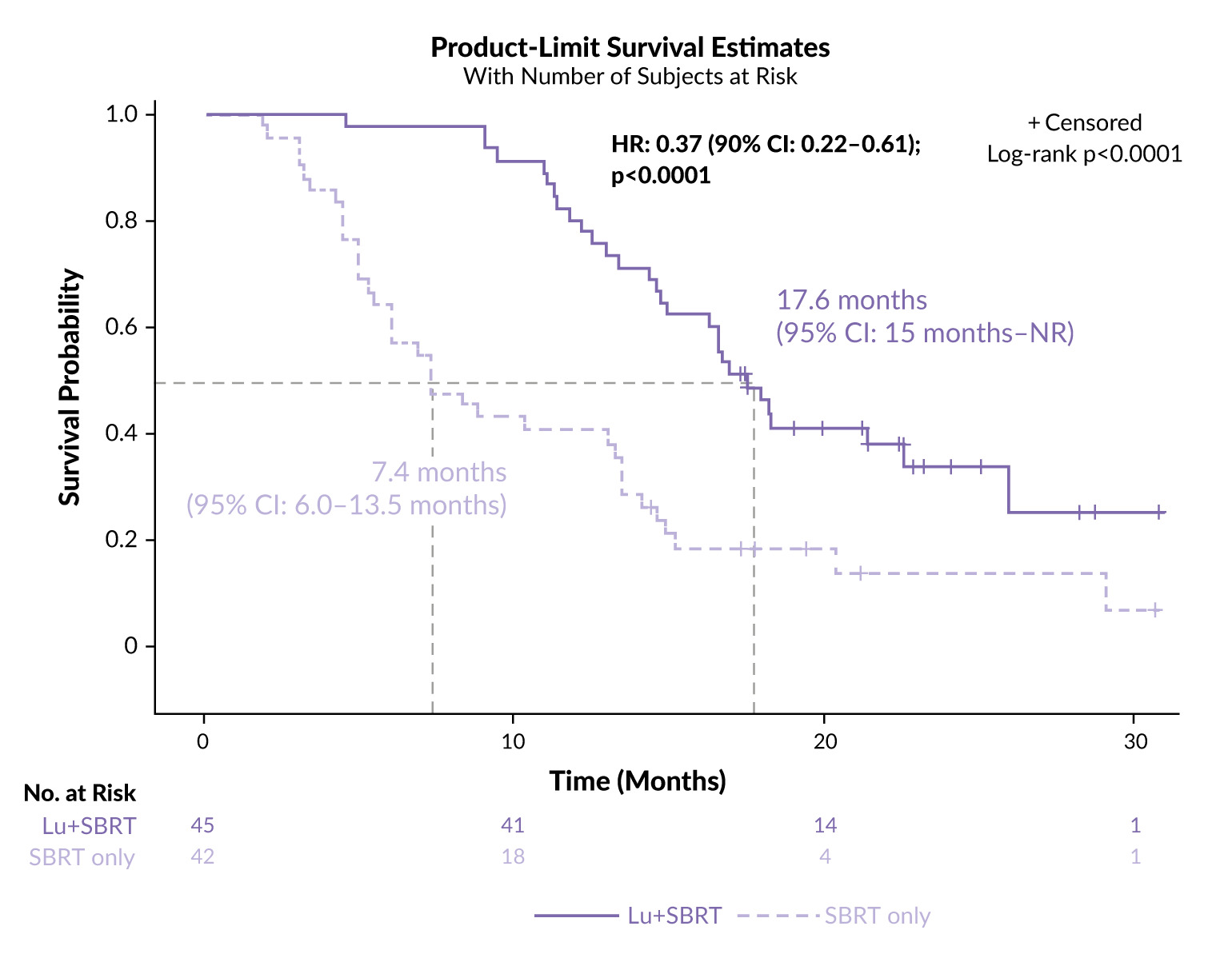
At a median follow-up of 22 months, neoadjuvant [177Lu]Lu-PNT2002 plus SBRT resulted in a 63% reduction in the risk of recurrence, need for ADT or death compared with SBRT alone (HR: 0.37 [95% CI: 0.22–0.61]; p<0.0001), with roughly 20% fewer patients showing progression (64% vs 86%).25 The median PFS was 17.6 months in the RLT arm compared with 7.4 months in the control arm, corresponding to an absolute gain of 10.2 months with [177Lu]Lu-PNT2002 (Figure 2). The PFS benefit was consistent across all subgroups.
Neoadjuvant [177Lu]Lu-PNT2002 showed a favorable safety profile owing to the low dose, with mostly low-grade AEs.25 There was a slightly higher incidence of dry mouth, dry eyes, gastrointestinal (diarrhea and constipation) and bone marrow toxicity in the RLT arm than in the control arm. Grade 3 AEs were limited to lymphopenia (6.7% vs 4.8%, respectively).
In summary, low-dose [177Lu]Lu-PSMA RLT with 6.8 GBq [177Lu]Lu-PNT2002 twice before SBRT demonstrated a significant PFS improvement in patients with oligo-recurrent HSPC and was well-tolerated.25 The effect was likely driven by eradication of occult metastatic disease. Still, there is potential for further optimization of the RLT regimen, as 64% of patients in the [177Lu]Lu-PNT2002 arm experienced disease progression. Notably, adding short-term ADT to radiotherapy has proven effective in cases of biochemical recurrence and, more recently, in oligometastatic disease (RADIOSA, EXTEND, SBRT-SG 05), where it is increasingly adopted as a standard treatment.26–31 Therefore, the choice of SBRT alone as the control arm does not fully reflect current clinical practice.
[177Lu]Lu-PSMA-617 plus ADT and ARPI in mHSPC
[177Lu]Lu-PSMA-617 has significantly improved disease outcomes in patients with PSMA-positive mCRPC after ARPI progression, both in the taxane-naïve setting (PSMAfore)18,19 and after prior taxane therapy (VISION).2 The phase III PSMAddition trial investigated its safety and efficacy in patients with metastatic HSPC (mHSPC).32 A total of 1,144 patients with previously untreated or minimally treated PSMA-positive mHSPC were randomly assigned at a 1:1 ratio to receive either [177Lu]Lu-PSMA-617 (7.4 GBq ± 10% Q6W for six cycles) in combination with ADT and ARPI or ADT plus ARPI alone. The primary endpoint was rPFS, with OS as a key secondary endpoint. Overall, 567 patients received treatment in the RLT arm and 562 in the control arm; 15.9% crossed over to [177Lu]Lu-PSMA-617 upon confirmed radiographic progressive disease (rPD).
At a median follow-up of 23.6 months, the [177Lu]Lu-PSMA-617 combination therapy significantly prolonged rPFS compared with ADT and ARPI alone (HR: 0.72 [95% CI: 0.58–0.90]; p=0.002), with 24.3% versus 30.1% of patients, respectively, experiencing rPD or death.32 The median rPFS was not reached in either arm.
Although showing favorable efficacy, the [177Lu]Lu-PSMA-617 combination was associated with a decrease in health-related quality of life (HRQoL). Unusual for [177Lu]Lu-PSMA-617,2,33 there was a persistent deterioration in the Functional Assessment of Cancer Therapy – Prostate (FACT-P) total score after eight months in the RLT arm compared with the control arm (median time to worsening, 11.33 months vs 17.12 months; HR: 1.14 [95% CI: 0.98–1.33]). More patients receiving the [177Lu]Lu-PSMA-617 combination therapy experienced any-grade and grade ≥3 hematological toxicity versus ADT plus ARPI alone, including anemia (any grade, 28% vs 14%; grade ≥3, 5% vs 2.8%), neutropenia (any grade, 14.7% vs 4.2%; grade ≥3, 3.7% vs 0.9%) and thrombocytopenia (any grade, 11.2% vs 3.4%; grade ≥3, 1.8% vs 0.5%). Higher rates of any-grade dry mouth (45.7% vs 3.7%) and gastrointestinal toxicities were also observed, including nausea (34.2% vs 9.4%), vomiting (13.8% vs 3.7%), and decreased appetite (14.4% vs 6.5%). The persistent separation of the Kaplan-Meier curves for time to deterioration in the FACT-P total score observed after approximately four cycles of [177Lu]Lu-PSMA-617 may reflect the absence of PSMA-positive disease in later cycles and increased radiotracer uptake in the parotid glands and small bowel.
In summary, adding [177Lu]Lu-PSMA-617 to ADT and ARPI demonstrated a statistically significant improvement in rPFS in patients with mHSPC, with increased toxicity contributing to a reduction in FACT-P score in the combination arm.32 As survival continues to improve in men with prostate cancer, maintaining an optimal HRQoL becomes essential. Since the trial employed the same dose as VISION,2 where patients were treated at a much later stage, further investigation is needed to determine which patient groups would derive the greatest benefit from the treatment and whether [177Lu]Lu-PSMA-617 (7.4 GBq × 6) is appropriate as a standard of care for all patients in this setting.
Associations between baseline quantitative PSMA-PET parameters and [177Lu]Lu-PSMA-617 efficacy: Secondary analysis of PSMAfore
In the phase III PSMAfore trial, [177Lu]Lu-PSMA-617 prolonged rPFS compared with the change of ARPI in taxane-naive adults with PSMA-positive mCRPC whose disease had progressed on a prior ARPI.18 However, no difference in OS between the treatment arms was demonstrated in the final analysis (HR: 0.91 [95% CI: 0.72–1.14]) which was confounded by high rates (60.3%) of crossover to the [177Lu]Lu-PSMA-617 arm.19 A recently presented secondary analysis of the trial aimed to assess the associations between baseline quantitative PSMA-PET parameters and [177Lu]Lu-PSMA-617 efficacy.34
In PSMAfore, 468 patients were randomized in a 1:1 ratio to [177Lu]Lu-PSMA-617 (7.4 GBq Q6W for six cycles) or ARPI change (abiraterone or enzalutamide).18 PSMA-positive mCRPC was defined as ≥1 PSMA-positive lesion and no exclusionary PSMA-negative lesions on baseline [68Ga]Ga-PSMA-11 PET/CT scans. In the current post hoc analysis, quantitative PET parameters including SUVmean, PSMA-positive tumour volume (TV), SUVmax, tumour load and presence of PSMA-positive lesions were extracted for bone, lymph node, liver, soft tissue and whole body.34
The study demonstrated that [177Lu]Lu-PSMA-617 improved rPFS compared with ARPI change, independent of SUVmean.34 However, higher whole-body SUVmean correlated with superior outcomes, although no optimal cut-off was identified. rPFS benefit was observed in both the SUVmean ≥median and SUVmean <median subgroups, with a greater effect in the higher SUVmean group. SUVmean was predictive of response to [177Lu]Lu-PSMA-617 versus ARPI. Similar trends were observed for OS, although the interpretation was limited by high crossover rates. Higher PSMA-positive tumor volume was associated with poorer outcomes across the treatment arms and served as a prognostic indicator of rPFS, irrespective of therapy. The therapeutic advantage of [177Lu]Lu-PSMA-617 over ARPI was more evident in patients with soft tissue-only mCRPC than in those with bone-only disease, which was likely due to the higher SUVmean and lower PSMA-positive TV in this subgroup.
In summary, the study demonstrated that SUVmean was associated with better outcomes in the [177Lu]Lu-PSMA-617 arm and PSMA-positive TV with worse outcomes in both arms.34 Overall, these data align with quantitative PSMA-PET findings from the VISION trial.2
AlphaBet: Combination of 223Ra and [177Lu]Lu-PSMA-617 in patients with mCRPC
For patients with mCRPC progressing following docetaxel, life-prolonging treatment options include [177Lu]Lu-PSMA-617 and the bone-seeking α-emitter 223Ra (for bone metastases).16 The phase I/II AlpaBet trial evaluated the safety of combining these two therapies.35 Eligible patients included men with progressive mCRPC after ARPI, at least two untreated bone lesions on bone scan, PSMA-PET positive disease (SUVmax ≥20) with no discordance (18-fluorodeoxyglucose [18F]FDG-positive with minimal PSMA and bone scan uptake) and prior exposure to ≥1 ARPI. Patients received [177Lu]Lu-PSMA-617 (7.4 GBq Q6W) and 223Ra (27.5 kBq/kg or 55 kBq/kg Q6W) for up to six cycles. The co-primary endpoints were the maximum tolerated dose (MTD) and recommended phase II dose (RP2D), as well as the rate of prostate-specific antigen (PSA) response, categorized by PSA decline of at least 50% (PSA50) or 90% (PSA90). Nine patients were treated in the dose escalation phase and 27 were included in the dose expansion cohort. The MTD and RP2D was 55 kBq/kg.
At a median follow-up of 13.3 months, there were no dose-limiting toxicities.35 Grade ≥3 TRAEs occurred in 14% of patients. The rates of PSA50 and PSA90 were 55% and 18%, respectively, and the median rPFS and PSA-PFS were 10 months and 5.3 months, respectively.
In summary, combining 223Ra with [177Lu]Lu-PSMA-617 was not associated with additional toxicity, and the efficacy outcomes were comparable to those observed in VISION.2,35 Careful selection of patients with a higher tumor burden could make this approach highly promising.
TheraPb: [212Pb]Pb-ADVC001 in patients with PSMA-positive mCRPC
The phase I/II TheraPb trial investigated the safety of [212Pb]Pb-ADVC001, a novel PSMA-targeting RLT employing α-particle emission.36 The study enrolled 22 patients with PSMA-positive mCRPC previously treated with at least one ARPI and at least one taxane, unless unsuitable or declined, with no prior RLT, except for 223Ra. The primary endpoints were safety and defining the RP2D. Escalating doses of 60–200 MBq were administered for up to six cycles at prespecified intervals. Cohort 1 received 60 MBq Q6W for four cycles; cohorts 2a, 3a and 4a received 120 MBq, 160 MBq and 200 MBq Q4W, respectively; cohorts 3b and 4b were optional continuation at Q2W and cohort 4c at Q1W, all for 4–6 cycles.
There were no dose-limiting toxicities or treatment-related serious AEs and no dose modifications or treatment discontinuations due to TRAEs (grade 3, 9%; grade 4–5, 0%).36 The most common TEAE was dry mouth (grade 1, 59%; grade 2, 23%; grade ≥3, 0%). There was rapid renal clearance and low uptake in normal organs, including the salivary glands. Most patients (12/15) experienced PSA50 responses at doses of at least 160 MBq.
In summary, [212Pb]Pb-ADVC001 demonstrated encouraging safety, despite the high doses used, along with promising anti-tumor response.36
LuPARP: Combining [177Lu]Lu-PSMA-617 with olaparib in patients with mCRPC
LuPARP is the first study to evaluate the safety and preliminary efficacy of [177Lu]Lu-PSMA-617 combined with olaparib in mCRPC.37 The rationale for pairing RLT with poly(ADP-ribose) polymerase (PARP) inhibitors is based on their complementary mechanisms of action. [177Lu]Lu-PSMA-617 induces DNA single-strand breaks that are repaired via PARP-dependent base excision repair, while inhibiting PARP converts these lesions into lethal double-strand breaks, thereby enhancing radiosensitivity.38 Preclinical data support the use of PARP inhibitors such as olaparib to potentiate RLT efficacy.
This phase I study employed a 3+3 dose-escalation design followed by dose expansion, enrolling 54 patients with high PSMA expression (SUVmax ≥15) and no discordant FDG-positive/PSMA-negative lesions.37 Patients received [177Lu]Lu-PSMA-617 (7.4 GBq every 6 weeks for up to six cycles) plus olaparib across ten dose levels (DL): DL1–6, 50–300 mg twice daily (BD) on days 2–15; DL7, 200 mg BD on days –4 to 14; DL8, 300 mg BD on days –4 to 14; DL9, 300 mg BD on days –4 to 18; and DL10, 300 mg BD on days –4 to 42. The primary endpoints were safety and dose-limiting toxicity. The secondary endpoints included rPFS, PSA response rate, PSA-PFS, ORR and OS.
After a median follow-up of 19 months, intermittent olaparib dosing (Day –4 cohorts) combined with [177Lu]Lu-PSMA-617 showed promising antitumor activity with manageable toxicity.37 The recommended phase II dose was established as DL9. At this level, the median rPFS was 12.8 months, the median OS was not yet estimable and the PSA90 response rate reached 55%. Across DL7–DL9, the median OS was 33.5 months, which compared favorably with the outcomes of the VISION trial. Continuous daily olaparib dosing was associated with increased myelosuppression, supporting the feasibility and tolerability of the intermittent dosing strategy for phase II evaluation.
[68Ga]-OncoACP3-DOTA and [177Lu]Lu-OncoACP3-DOTA for imaging and treatment of prostate cancer
Prostatic acid phosphatase (ACP3) is highly and almost exclusively expressed in prostate tumor cells, providing a rationale for its targeting in prostate cancer patients.39 In the first-in-human retrospective analysis of 27 PET scans from 25 patients using [68Ga]Ga-OncoACP3-DOTA matched with PSMA-targeted PET, tracer uptake was noted in all patients, and in 55% of scans the diagnostic performance of [68Ga]Ga-OncoACP3-DOTA was superior to the PSMA comparator.40 The biodistribution was favorable with significantly lower uptake in the liver, kidney, parotid and small intestine compared to PSMA PET (all p<0.002). Early therapeutic use of [177Lu]Lu-OncoACP3-DOTA in the first treated patients was feasible and safe. These findings support the further development of OncoACP3-DOTA for both imaging and radioligand therapy in prostate cancer.
Future outlook and conclusions
Radioligand therapy has entered a phase of rapid clinical maturation, supported by growing evidence across different tumor types. Established β-emitters, such as [177Lu]Lu-PSMA-617, continue to define standards in prostate cancer, while α-emitting compounds, such as [212Pb]Pb-DOTAMTATE, demonstrate meaningful activity and tolerability in GEP-NETs. The expanding landscape now includes combination strategies such as RLT with PARP inhibition, integration with stereotactic body radiotherapy and exploration of alternative targets such as ACP3 to overcome resistance and heterogeneity. Together, these advances highlight the transition from proof-of-concept to broad clinical applications. The continued refinement of patient selection, optimization of dosing and sequencing and the development of next-generation ligands will be central to extending the benefits of RLT across tumor types and disease stages.
Conflict of interest
Irene A. Burger has received research grants and speaker honoraria from GE Healthcare, research grants from Vontobel foundation and Bayer, and speaker honoraria from Bayer Health Care, Novartis, Jansen and Astellas Pharma AG and advisory roles for GE Healthcare, Novartis and RatioTherapeutics.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.