EGFR-mutated NSCLC: Oncogenic driver mutations and treatment guidelines
Non-small cell lung cancer (NSCLC) is the predominant type of lung cancer, accounting for approximately 85% of all cases.1,2 Among the genetic alterations associated with NSCLC, activating mutations in the epidermal growth factor receptor (EGFR) gene are present in 10–15% of cases, with a higher incidence reported in specific patient populations, such as never-smokers, females, younger individuals and those of Asian descent.3 Most EGFR mutations in NSCLC fall into two main categories: in-frame deletions in exon 19 (ex19del) and L858R substitutions in exon 21, collectively known as common mutations, which account for approximately 85–90% of all EGFR-altered tumors.4 Additionally, insertions in exon 20 represent up to 12% of EGFR mutations. Identifying these specific mutations is critical for guiding treatment decisions as they can directly influence the response to targeted therapies, particularly to EGFR tyrosine kinase inhibitors (TKIs).5 Due to an altered conformation at the kinase binding site, tumors with EGFR exon 20 insertions are largely resistant to TKI therapy.
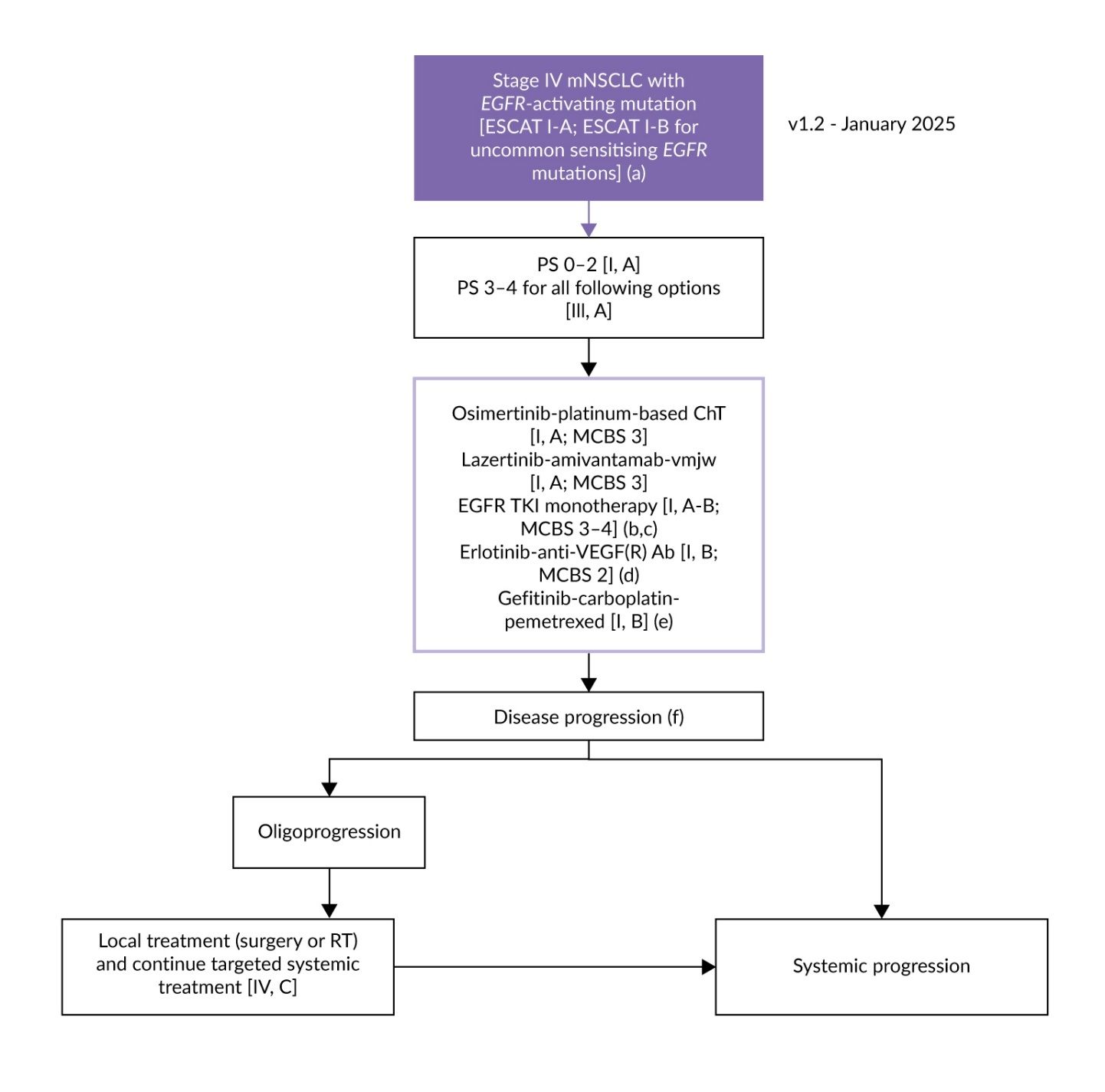
According to the European Society for Medical Oncology (ESMO) guidelines, patients with locally advanced or metastatic NSCLC whose tumors harbor a classical EGFR mutation should receive treatment with an EGFR TKI, irrespective of clinical characteristics.1,6 Third-generation TKIs, such as osimertinib or lazertinib, are the preferred option (Figure 1). The recommended regimens include osimertinib with or without chemotherapy and lazertinib in combination with amivantamab, an EGFR- and mesenchymal-epithelial transition factor (MET)-directed bispecific monoclonal antibody.
Amivantamab in treatment of EGFR-mutated NSCLC
Bispecific antibodies represent a significant advancement in cancer therapy owing to their innovative structural design that allows them to simultaneously target multiple receptors or immune pathways and exert synergistic antitumor effects through diverse and complementary mechanisms.9 Amivantamab is a fully human EGFR- and MET-targeting bispecific antibody with multiple mechanisms of action, including inhibition of ligand binding, receptor degradation and immune cell-directed activity.10–13 These combined actions help to overcome resistance to TKIs in tumors harboring EGFR exon 20 insertions, address MET-mediated resistance and potentially improve antitumor immune responses.13–16
Amivantamab was initially investigated in patients with NSCLC harboring EGFR exon 20 insertions.17 First approved as monotherapy following progression on platinum-based chemotherapy, the combination of amivantamab with carboplatin and pemetrexed is currently the preferred first-line regimen in this setting.1,6,8,18 In the phase III PAPILLON trial, the combination significantly prolonged progression-free survival (PFS) compared with chemotherapy alone (HR: 0.40 [95% CI: 0.30‒0.53]; p<0.001).19 The same regimen has also been approved for patients with NSCLC harboring classical EGFR mutations who experience disease progression on osimertinib, based on data from the phase III MARIPOSA-2 study, which demonstrated a significant PFS benefit versus chemotherapy alone (HR: 0.48 [95% CI: 0.36‒0.64]; p<0.001).18,20
In the frontline setting, the chemotherapy-free combination of amivantamab with lazertinib is recommended by both ESMO and National Comprehensive Cancer Network (NCCN) guidelines (category 1), based on the results from the MARIPOSA trial.1,8,21–23
First-line amivantamab plus lazertinib in patients with EGFR-mutated advanced NSCLC: OS updates from MARIPOSA
In the phase III MARIPOSA study, 1,074 treatment-naïve patients with locally advanced or metastatic EGFR-mutated (ex19del/L858R) NSCLC were randomized in a 2:2:1 ratio to receive either amivantamab plus lazertinib, osimertinib alone or lazertinib alone.22 The primary analysis demonstrated superior PFS with amivantamab plus lazertinib over SoC osimertinib at a median follow-up of 22 months (HR: 0.70 [95% CI: 0.58–0.85]; p<0.001). As assessed by blinded independent central review, the median PFS was 23.7 months in the combination arm versus 16.6 months in the osimertinib arm, with 2-year PFS rates of 48% and 34%, respectively.
More recent data from the protocol-specified final overall survival (OS) analysis further support the clinical benefit of amivantamab plus lazertinib.24,25 At a median follow-up of 37.8 months, the combination regimen showed a statistically significant and clinically meaningful improvement in OS compared with osimertinib monotherapy (HR: 0.75 [95% CI: 0.61–0.92]; p=0.005), continued curve separation and an estimated OS benefit exceeding one year (median, not reached vs 36.7 months) (Figure 2). The 3-year OS rates were 60% versus 51%, respectively. Notably, superior OS with amivantamab plus lazertinib was consistently observed across all patient subgroups, including sex, race, EGFR mutation type (ex19del or L858R), Eastern Cooperative Oncology Group (ECOG) performance status and history of brain metastases. Furthermore, updated analysis of the study demonstrated that amivantamab plus lazertinib significantly reduced the development of EGFR- and MET-driven resistance compared with osimertinib, with no significant upregulation of other resistance pathways.26
In terms of safety, skin-related adverse events (AEs) were more frequently reported with amivantamab plus lazertinib compared with osimertinib, including grade ≥3 rash (17% vs 1%), paronychia (12% vs <1%) and acneiform dermatitis (9% vs 0%).22 The combination therapy was also associated with a higher rate of infusion-related reactions (IRRs) (any-grade, 65% vs 0%; grade ≥3, 6% vs 0%) and pulmonary embolism (any-grade, 19% vs 6%; grade ≥3, 9% vs 3%).
Managing amivantamab-associated toxicity
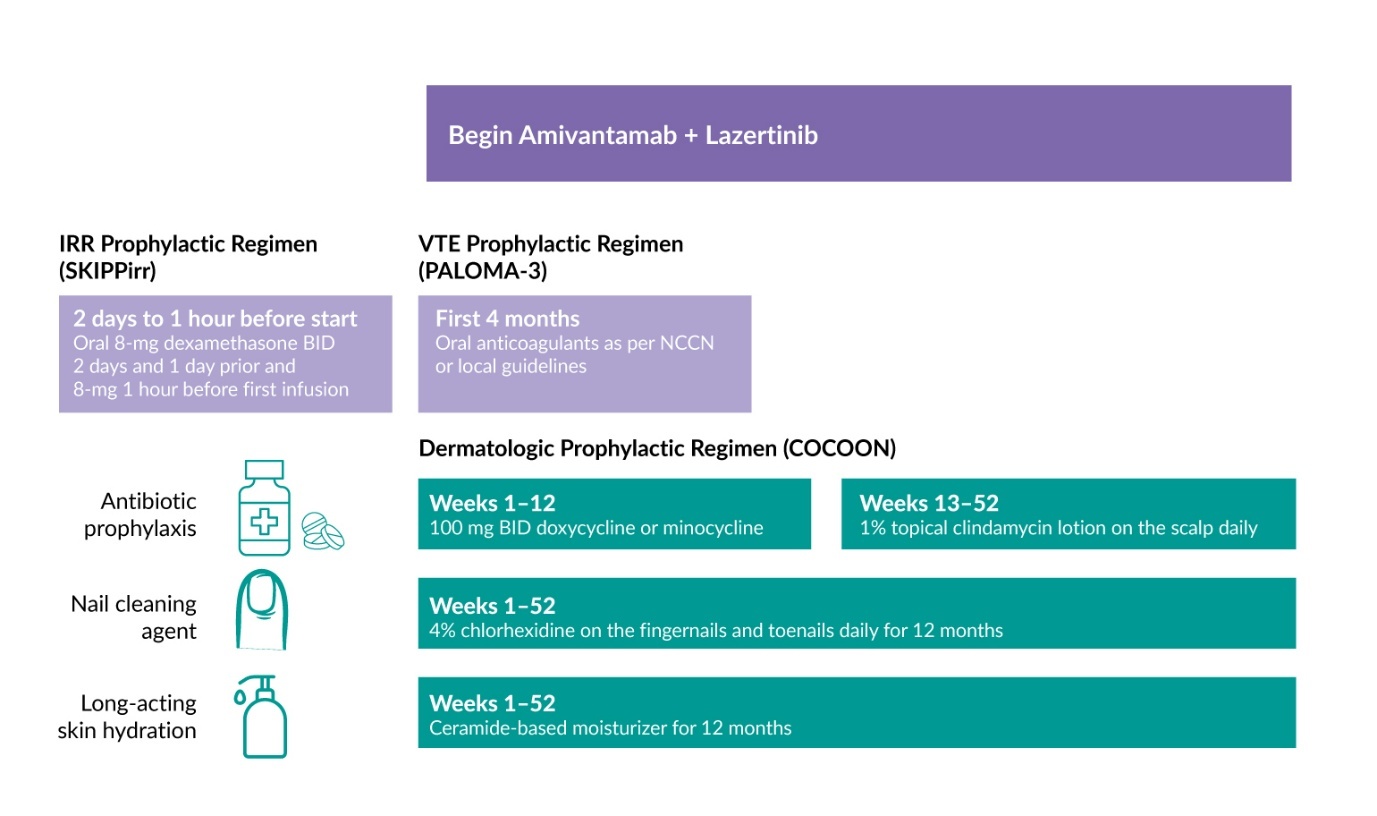
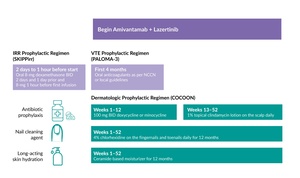
Dermatologic AEs such as rash and paronychia are common with EGFR inhibitors and are often managed reactively. However, early and proactive management may help patients maintain the treatment longer. Several recent studies have investigated proactive therapeutic strategies to reduce toxicity associated with the combination of amivantamab and lazertinib. In the phase II COCOON trial, 200 patients treated with amivantamab and lazertinib as first-line therapy for EGFR-mutated NSCLC (ex19del/L858R) were randomized to receive enhanced dermatologic management or standard dermatologic management.27 The enhanced regimen included oral doxycycline/minocycline administered at a dose of 100 mg twice daily for 12 weeks, followed by a clindamycin 1% lotion for the scalp between week 13 and 52 (Figure 3). Patients also received chlorhexidine 4% wash for nail care and a ceramide-based noncomedogenic skin moisturizer for the body and face at least once daily for 12 months. The primary endpoint was the incidence of grade ≥2 dermatologic AEs of interest (DAEIs) within the first 12 weeks of treatment.
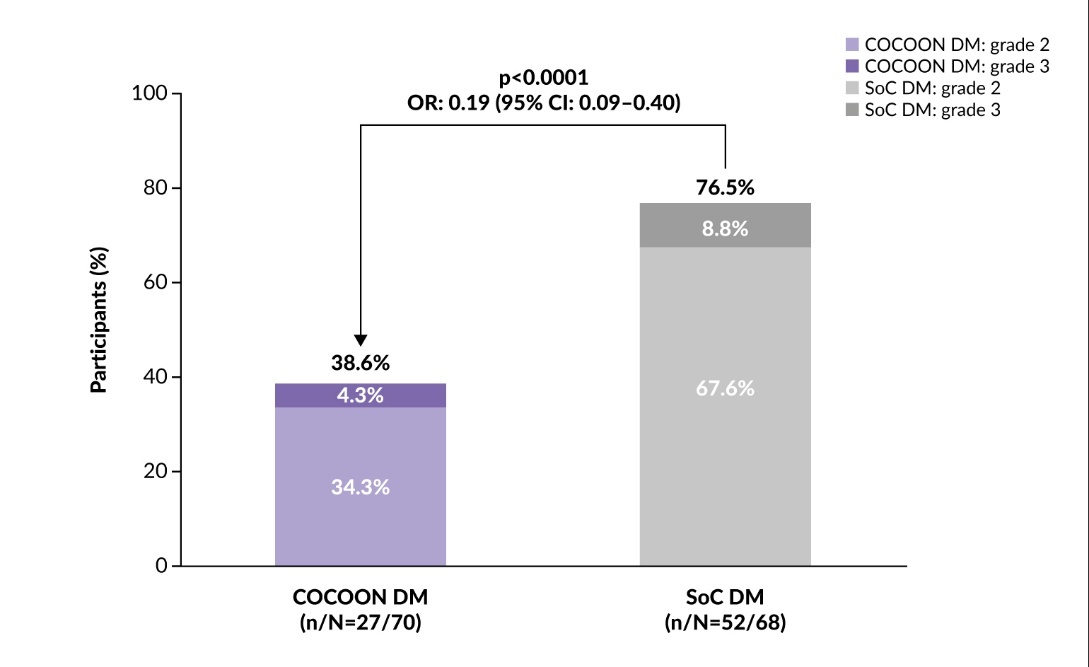
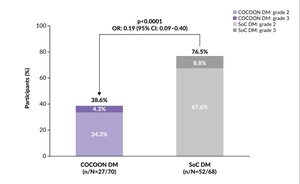
The study met its primary endpoint, showing that enhanced dermatologic management significantly reduced the incidence of grade ≥2 DAEIs. Within the first 12 weeks of treatment, 38.6% of patients in the enhanced dermatologic management arm experienced grade ≥2 DAEIs compared with 76.5% of patients in the SoC arm (odds ratio [OR]: 0.19 [95% CI: 0.09–0.40]; p<0.0001) (Figure 4), with a three-fold reduction in the number of patients with two or more different grade ≥2 DAEIs (6% vs 18%).27 The enhanced dermatologic management was also associated with a two-fold reduction in the incidence of grade 3 DAEIs (4.3% vs 8.8% with SoC). The trial demonstrated that proactive dermatologic management significantly reduced both the frequency and severity of dermatologic AEs, potentially encouraging adherence to treatment and improving patient outcomes.
The phase II SKIPPirr trial investigated strategies to prevent IRRs in patients with previously treated advanced or metastatic NSCLC with classical EGFR mutations who were receiving the combination of amivantamab and lazertinib.28 The study evaluated four distinct prophylactic approaches, each administered along with standard pretreatment with antihistamines, antipyretics and 10 mg intravenous (IV) dexamethasone. Using Simon’s two-stage design, the study included expansion-phase regimens that demonstrated sufficient efficacy in the initial stages. The primary endpoint was the incidence of IRR on cycle 1 day 1 (C1D1), the time point when most IRRs typically occur.
Among the strategies tested, a five-dose regimen using dexamethasone proved most effective.28 This regimen involved administering 8 mg oral dexamethasone twice daily on days -2 and -1 before cycle 1 (C1D-2 and C1D-1) and one dose one hour before amivantamab infusion (Figure 3). This approach achieved a three-fold reduction in IRRs compared with data from the CHRYSALIS study (22.5% vs 67.4%).
Additionally, the phase III PALOMA-3 trial investigated subcutaneous (SC) versus IV administration of amivantamab plus lazertinib in patients with locally advanced or metastatic EGFR-mutated (ex19del/L858R) NSCLC who progressed after osimertinib and platinum-based chemotherapy.29 In terms of efficacy, the SC formulation demonstrated noninferiority to the IV route, with comparable ORR (30% vs 33%) and median PFS (6.1 months vs 4.3 months). Notably, OS was significantly longer in the SC versus IV group (HR: 0.62 [95% CI: 0.42–0.92]; p=0.02). The SC formulation was also associated with a markedly lower incidence of IRRs. Any-grade IRRs occurred in only 13% of patients receiving SC amivantamab compared with 66% of patients receiving IV amivantamab, with grade ≥3 events reported in 0.5% versus 4%, respectively.
Given the elevated risk of venous thromboembolism (VTE) in patients with lung cancer, the NCCN guidelines recommend prophylactic anticoagulation at the time of therapy initiation.21,22 Therefore, the PALOMA-3 study protocol was amended to include prophylactic anticoagulation for the first four months of treatment (Figure 3), as most VTEs occur during this period. With approximately 80% of patients in both groups receiving anticoagulant prophylaxis, the incidence of VTE was reduced from 21% to 10%. Among those receiving prophylactic anticoagulation, the SC arm showed a lower rate of VTEs compared with the IV arm (7% vs 12%).
Osimertinib in patients with EGFR-mutated NSCLC
Osimertinib monotherapy or a combination with platinum and pemetrexed is another first-line treatment option for patients with EGFR-mutated NSCLC.
In the phase III FLAURA trial, osimertinib demonstrated significant survival benefit over first-generation EGFR TKIs gefitinib and erlotinib, with a median PFS of 18.9 months versus 10.2 months (HR: 0.46 [95% CI: 0.37–0.57]; p<0.001) and a median OS of 38.6 months versus 31.8 months (HR: 0.80 [95% CI: 0.64‒1.00]; p=0.046).30,31
Osimertinib has been further studied in combination with chemotherapy in the FLAURA 2 trial.32 In this study, 557 patients were randomized 1:1 to receive either osimertinib in combination with pemetrexed and platinum-based chemotherapy or osimertinib alone. Treatment with osimertinib plus chemotherapy led to significant PFS improvement (median 25.5 months vs 16.7 months; HR 0.62 [95% CI: 0.49–0.79]; p<0.001),33 and OS improvement (median OS. 47.5 months vs 37.6 months; HR: 0.77 [95% CI: 0.61-0.96]; p=0.0202) compared with osimertinib alone.34 The findings were consistent across most predefined patient subgroups, including sex, age, EGFR mutation type, World Health Organization (WHO) performance status and central nervous system (CNS) involvement at baseline. Hazard ratios for PFS favoring osimertinib plus chemotherapy were 0.59 (95% CI: 0.40–0.87) in patients with CNS involvement and 0.89 (95% CI: 0.62–1.28) in those without.33 In terms of safety, grade ≥3 AEs occurred in the 64% of the patients in the osimertinib–chemotherapy group and in 27% of the patients receiving osimertinib monotherapy. Expectedly, most grade≥3 AEs in the combination group were hematologic (71%).
Choosing between first-line options for metastatic EGFR-mutated NSCLC
Amivantamab-lazertinib and osimerinib-chemotherapy improve PFS and prolong OS, and thus represent standard first-line options. However, both are associated with increased toxicities compared to osimertinib alone. Results from the COCOON, SKIPPirr and PALOMA-3 trials offer significant improvements in the toxicity profile of amivantamab and lazertinib, which can facilitate their tolerability; however, this comes at the cost of a demanding supportive care regimen involving corticosteroids, antibiotics, anticoagulants and skincare. On the other hand, some patients may still exhibit long-term disease control with osimertinib monotherapy, which is a convenient oral and well-tolerated regimen. When selecting first-line therapy, several factors should thus be considered: the presence or absence of poor-prognostic factors and patients’ preferences regarding the balance between efficacy and toxicity. Some poor-prognostic factors, such as brain or liver metastases, L858R mutation or the presence of detectable circulating tumor DNA, are known to be associated with poorer outcomes on osimertinib monotherapy. While these factors are prognostic rather than predictive of improved benefit from combination approaches, both combination regimens have proven superior to osimertinib monotherapy in these settings.35 In such scenarios, which represent a majority of patients, combination approaches should probably be the preferred options. Importantly, however, the patient’s preferences regarding the balance between treatment efficacy and toxicities/constraints also represent an essential consideration. Moreover, further clinical and translational research is needed to clarify which subsets of patients will benefit most from amivantamab and lazertinib versus osimertinib and chemotherapy.
Conclusions
The therapeutic landscape for previously untreated EGFR-mutated (ex19del/L858R) locally advanced or metastatic NSCLC is evolving, with new combination regimens offering promising outcomes. The combinations of amivantamab and lazertinib, and of osimertinib and chemotherapy demonstrated a significant survival benefit compared with osimertinib alone in this patient population, but they were associated with higher rates of AEs. Despite this, proactive supportive care can effectively manage and mitigate treatment-related side effects, highlighting the importance of multidisciplinary management and active patient engagement in improving clinical outcomes. The combinations of amivantamab and lazertinib and osimertinib and chemotherapy are now approved by Swissmedic as first-line regimens for adult patients with locally advanced or metastatic EGFR-mutated (ex19del/L858R) NSCLC.18
Conflict of interest
M.B. received financial support to attend scientific meetings for registration, travel and accommodation from Bayer, MSD, AstraZeneca and PharmaMar. A.M. received financial support to attend scientific meetings for registration, travel and accommodation from Johnson&Johnson, Roche and PharmaMar. A.A declares consultation fees from Bristol-Myers Squibb, AstraZeneca, Roche, MSD, Pfizer, Eli Lilly, Astellas, Amgen, Novartis, Merk and Johnson&Johnson; is on the speaker bureau for Eli Lilly, AstraZeneca and Amgen; and has received research grants to the institution from AstraZeneca.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
All authors have contributed to and approved the final manuscript.
_in_the_mariposa_trial.png)
_in_the_mariposa_trial.png)