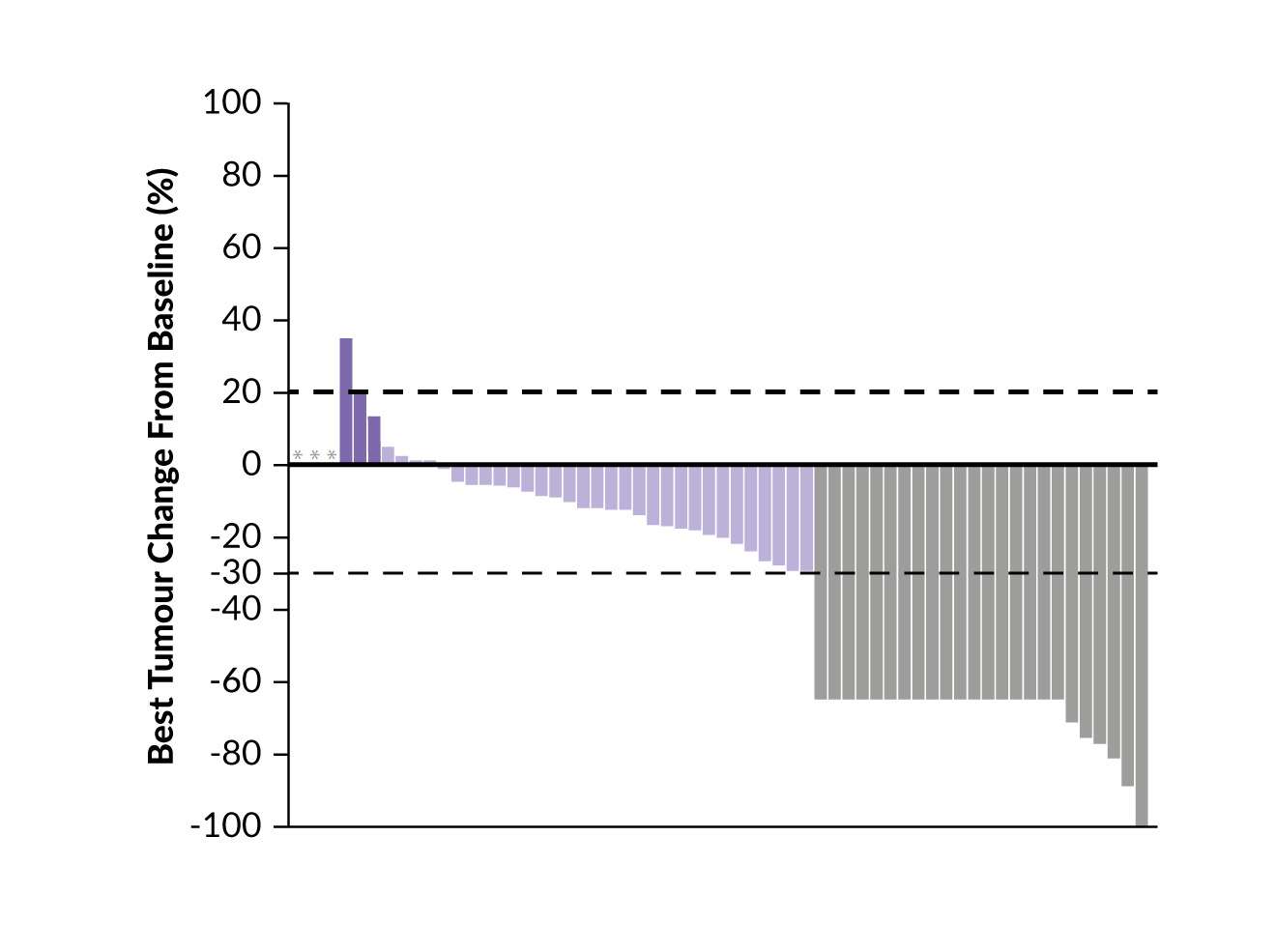
SAKK 16/14: Perioperative durvalumab plus neoadjuvant chemotherapy in patients with resectable stage IIIA(N2) NSCLC
Neoadjuvant chemotherapy with cisplatin and docetaxel has been a standard of care for patients with resectable stage IIIA(N2) non-small cell lung cancer (NSCLC).1 In the primary analysis of the multicenter, single-arm, phase II trial SAKK 16/14, the addition of perioperative durvalumab to neoadjuvant chemotherapy resulted in a major pathologic response rate (MPR, defined as ≤10% vital tumor cells in the primary tumor) of 62% and a 1-year event-free survival (EFS) rate of 73% (median EFS of not reached). At the European Lung Cancer Congress (ELCC) 2025, Prof. Sacha Rothschild from the Cantonal Hospital Baden presented the final analysis of this trial.2
The trial enrolled 68 patients with resectable, locally advanced T1–3N2M0, stage IIIA(N2) (6th edition TNM staging) NSCLC.2 Involvement of mediastinal lymph nodes (N2 disease) was cytologically proven. The patients received chemotherapy consisting of three cycles of cisplatin (100 mg/m2) and docetaxel (85 mg/m2), followed by two cycles of durvalumab (750 mg every two weeks). After resection, adjuvant durvalumab was administered for 12 months. The primary endpoint was EFS at 12 months, with the hypothesis for statistical considerations being an improvement from 48% (based on data from the trial SAKK 16/00) to 65%. Secondary endpoints included EFS, overall survival (OS), objective response rate (ORR), pathologic complete response (pCR) and MPR.
The median age was 61 years, with an age range of 41–74 years.2 Most patients had adenocarcinoma (55.2%) and squamous cell carcinoma (32.8%) histology. Overall, 22.4% of patients had stage T1 disease, 49.3% had stage T2 and 28.4% had stage T3 disease. Approximately half of the patients (47.8%) had tumor programmed death-ligand 1 (PD-L1) expression of ≥1%.
At the currently reported final analysis after a median follow-up of 72 months, the median EFS was 4 years, with a 5-year EFS rate of 45.9% (Figure 1).2 The median OS was not reached and the 5-year OS rate was 65.8%.
In the subgroup of patients achieving a pCR (n=10), the median EFS was not reached and the 5-year EFS rate was 71.4%.2 In these patients, the median OS was also not reached, with a 5-year OS rate of 100%. Similarly, in patients with MPR (n=34), both the median EFS and OS were not reached, with a 5-year EFS rate of 65.7% and a 5-year OS rate of 97%.
Tumor PD-L1 expression was demonstrated to be a predictive factor for response to perioperative durvalumab.2 The median OS was not reached in patients with tumor PD-L1 expression of at least 1% compared with 5.2 years (p=0.19) in those with PD-L1 below 1%.
All patients included in the safety analysis set (n=67) experienced at least one adverse event (AE) (grade ≥3, 88.1%) with neoadjuvant chemotherapy as the main driver of toxicity.2 There were two fatal AEs; one patient experienced respiratory failure during neoadjuvant chemotherapy and one patient had bronchopulmonary hemorrhage after surgery. The 30-day postoperative mortality rate was 1.8%.
In conclusion, SAKK 16/14 is the study with the longest reported follow-up of perioperative immunotherapy in resectable NSCLC. In the meantime, many randomized studies have confirmed this new standard of care, but the trial SAKK 16/14 remains the only one with results from a follow-up of more than 5 years.2 Results showed that adding perioperative durvalumab to standard chemotherapy was safe, with encouraging EFS and OS rates that exceeded historical data. Based on these results, the follow-up trial SAKK 16/18 is currently ongoing, in which the additional benefit of neoadjuvant immunomodulatory radiotherapy given at the same time as immunotherapy is being investigated.
Trial SAKK 17/18 ORIGIN: Efficacy and safety of gemcitabine plus atezolizumab in pretreated patients with pleural mesothelioma
Few treatment options are available for patients with pleural mesothelioma progressing on ICI or chemotherapy.3 A previous preclinical model indicated that combining ICI with gemcitabine can overcome resistance to ICI.4 The trial SAKK 17/18 ORIGIN is a multicenter phase II trial evaluating the activity of gemcitabine and atezolizumab in patients with pleural mesothelioma whose disease has progressed following ICI or chemotherapy. Dr Angelica Rigutto from the University of Fribourg presented results from this trial.5
Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 and inoperable pleural mesothelioma with documented recurrence or progression during or after at least one prior line of ICI and/or chemotherapy.5 Treatment consisted of gemcitabine (1,000 mg/m2 intravenously on days 1 and 8 every three weeks [Q3W]) and atezolizumab (1,200 mg intravenously Q3W). The primary endpoint was ORR in the full analysis set, assessed by independent radiological review using modified RECIST. Secondary endpoints included progression-free survival (PFS), disease control rate (DCR) at 18 weeks, duration of response (DoR), OS and safety. Proteomic profiling was performed on baseline plasma samples using Biognosys’ P2 enrichment workflow and data-independent acquisition mass spectrometry.
The trial enrolled 30 patients who received gemcitabine plus atezolizumab following a median of two prior lines of therapy (range, 1–5).5 The median age was 70 years (range, 43–81) and 86.7% of patients were male. The most common histological subtype was epithelioid (83.3%). Overall, 36.7% of patients had an ECOG PS of 0, while 60% had a PS of 1 and 3.3% had a PS of 2.
After a median follow-up of 24.1 months, the ORR was 13.3%, including a partial response (PR) rate of 13.3%.5 Half of the patients achieved stable disease, resulting in a DCR of 63.3%. The median DoR was 7.3 months and the DCR at 18 weeks was 43.3%. In terms of survival outcomes, the median OS was 9.1 months and the median PFS was 5.6 months (Figure 2). Treatment-related adverse events (TRAEs) occurred in 86.6% of patients, with grade 3–4 events in 33.3% and treatment discontinuation in 13% of patients.
Proteomic analysis identified over 8,500 circulating proteins, with 174 being differentially expressed between responders and non-responders. Pathway analysis highlighted neutrophil extracellular trap (NET) formation as the most significantly enriched process in responders. Furthermore, a 14-protein signature predictive of treatment response was identified.
In conclusion, the trial ORIGIN met its primary endpoint of ORR, demonstrating clinically meaningful activity of gemcitabine plus atezolizumab in patients with pretreated pleural mesothelioma. Additionally, a novel blood-based protein signature was identified, offering potential for predictive biomarker development.
ETOP AMAZE-lung: Amivantamab, lazertinib and bevacizumab in EGFR-mutant advanced NSCLC after progression on osimertinib
Therapeutic strategies targeting epidermal growth factor receptor (EGFR), MET and angiogenesis pathways have shown potential to overcome resistance to EGFR tyrosine kinase inhibitors (TKIs) in EGFR-mutant NSCLC.6 Amivantamab, a bispecific antibody directed against EGFR and MET, has demonstrated improved outcomes when combined with chemotherapy in patients with tumor progression on osimertinib, a third-generation EGFR TKI.7,8
ETOP AMAZE-Lung is a multicenter, single-arm, phase II trial designed to evaluate a chemotherapy-sparing strategy in patients with advanced EGFR-mutant NSCLC and acquired resistance to third-generation EGFR TKIs.9 Final results were presented by Dr Ross Soo from the National University Cancer Institute, Singapore, on behalf of a consortium that included several Swiss partners such as the Swiss Cancer Institute, Kantonsspital St. Gallen, Inselspital Bern, HUG Geneva, ETOP IBCSG Partners Foundation and Lausanne University Hospital (CHUV).
Eligible patients had chemotherapy-naïve, advanced non-squamous NSCLC with documented EGFR exon 19 deletion or L858R mutations and disease progression on third-generation EGFR TKIs.9 Patients received a combination of amivantamab (<80 kg: 1,750 mg; ≥80 kg: 2,100 mg; Q3W), lazertinib (240 mg daily) and bevacizumab (15 mg/kg Q3W) until disease progression or unacceptable toxicities. The primary endpoint was ORR at 12 weeks. The study was powered to detect a clinically meaningful improvement in ORR, with a target sample size of 60 patients providing 93% power to reject the null hypothesis of an ORR ≤20% versus 40%. Secondary endpoints included DCR, DoR, PFS, OS and safety.
A total of 61 patients were enrolled and 60 received at least one dose of study treatment.9 At a median follow-up of nine months, 48 patients remained alive. The 12-week ORR was 33% (20 confirmed responses), meeting the primary endpoint. Partial responses were observed in 24 of 61 patients (39%) (Figure 3). The DCR was 79% and the median DoR was 13.9 months. Furthermore, the median PFS was 10.9 months, with 3- and 6-month PFS rates of 80% and 64%, respectively. The median OS was 15.5 months and the 3- and 6-month OS rates were 95% and 88%, respectively.
The most common TRAEs included infusion-related reactions (58%), acneiform rash (50%), paronychia (42%) and fatigue (40%).9 TRAEs led to the discontinuation of one or more study drugs in 20% of patients. Grade 3–4 venous thromboembolism occurred in 3% of patients and no fatal TRAEs were reported.
In conclusion, the trial ETOP AMAZE-Lung met its primary endpoint, demonstrating that the combination of amivantamab, lazertinib and bevacizumab provides clinically meaningful antitumor activity with a manageable safety profile.9 These results support this regimen as a promising chemotherapy-sparing treatment option for patients with EGFR-mutant NSCLC who have progressed on third-generation EGFR TKIs.
ETOP 25–23 ADOPT-lung: Adjuvant durvalumab in resectable stage IIB–IIIB NSCLC
The incorporation of programmed cell death protein 1 (PD-1)/PD-L1 ICIs into perioperative treatment strategies has significantly improved EFS and OS in patients with early and locally advanced NSCLC.10 However, the optimal duration and scheduling of these therapies remain to be defined. Presented by PD Dr Sabine Schmid from Inselspital Bern, the trial ETOP 25–23 ADOPT-lungis an ongoing international, multicenter, open-label, randomized phase III trial conducted across 42 centers in 10 countries, including nine centers in Switzerland.11
The trial investigates the added clinical benefit of adjuvant durvalumab following neoadjuvant chemoimmunotherapy in patients with resectable stage IIB–IIIB (N2) NSCLC.11 Eligible patients must have histologically confirmed, treatment-naïve, resectable stage IIB–IIIB (T1–T4 (size) N0–N2) NSCLC according to the 8th edition of the TNM staging manual. Patients must have known PD-L1 status and no EGFR mutations or ALK rearrangements. Treatment includes 3–4 cycles of neoadjuvant durvalumab (1,500 mg Q3W) in combination with platinum-doublet chemotherapy, followed by surgery. Patients achieving R0 or R1 resection are randomized to receive either 12 cycles of adjuvant durvalumab (1,500 mg every four weeks) or to observation. Those with an R1 resection additionally receive adjuvant radiotherapy according to institutional guidelines. Randomization is stratified by pCR (pCR vs non-pCR), histological subtype (squamous vs non-squamous), disease stage (IIB vs III) and PD-L1 expression (<1% vs ≥1%), with a maximum of 40% of patients permitted to have PD-L1 expression below 1%.
The primary endpoint is disease-free survival (DFS) in patients without a pCR.11 Key secondary endpoints include DFS in the intention-to-treat (ITT) population, DFS in patients achieving pCR, OS and safety. The central hypothesis is that adjuvant durvalumab will improve DFS in patients who do not achieve pCR following neoadjuvant chemoimmunotherapy.
The target sample size is 290 patients without a pCR.11 To meet this goal, approximately 520 patients will be enrolled, with 364 expected to be eligible for randomization. The trial was activated on October 31, 2024, and as of March 24, 2025, four patients have been enrolled.
Preliminary insights into molecular predictors of response to first-line chemoimmunotherapy in ES-SCLC
For patients with extensive-stage small cell lung cancer (ES-SCLC), the current standard of care first-line treatment is a combination of platinum-based chemotherapy and ICI.12 Molecular subtyping of SCLC based on immunohistochemical markers, including mammalian achaete-scute homolog-1 (MASH1), neurogenic differentiation factor 1 (NEUROD1) and POU class 2 homeobox 3 (POU2F3), has emerged as a promising framework for better understanding tumor biology and guiding therapeutic strategies.13 Additionally, low expression of Schlafen 11 (SLFN11) has been associated with poor prognosis and may be a predictive biomarker for the response to several drugs. However, its predictive role in the context of chemoimmunotherapy remains unclear. A retrospective, multicenter study presented by Núria M. Zellweger from the University Hospital Basel assessed clinical outcomes with first-line chemoimmunotherapy according to immunohistochemical SCLC subtypes, SLFN11 expression and RNA-based T cell receptor (TCR) analysis.14
This real-world study included patients with ES-SCLC who received first-line ICI in combination with platinum-based chemotherapy at 10 cancer centers across Switzerland.14 Initial tumor biopsy samples were centrally collected and analyzed. Immunohistochemistry for MASH1, NEUROD1, PO2F3 and SLFN11 was performed using standard protocols. Total RNA was extracted for TCR sequencing using the Oncomine TCR SR Assays. Key immunological metrics, including molecular subtypes, TCR evenness, Shannon diversity and number of clones were evaluated for the association with PFS, OS and ORR using Cox regression models and Kruskal-Wallis tests.
Of 201 patients included, tumor tissue was available for 60 patients and to date, analyses have been performed on samples from 20 patients.14 Baseline patient characteristics of this subgroup were comparable to those of the full cohort. Trends toward improved OS were observed in patients with MASH1 expression (p=0.110), absence of NEUROD1 expression (p=0.121) and greater TCR evenness (p=0.186). Results further showed that a lower number of TCR clones (p=0.31) and lower Shannon diversity (p=0.14) were associated with a trend toward a higher ORR. SLF11 expression was detected in 55% of samples, with a higher ORR observed in SLFN11-positive cases compared with SLFN11-negative cases (69% vs 40%; p=0.33).
In summary, these preliminary findings suggest that immunohistochemical subtypes and TCR repertoire diversity may impact treatment outcomes with chemoimmunotherapy in patients with ES-SCLC.14 Ongoing analyses of additional tumor samples from the cohort are expected to provide further insights.
Conclusions
Recent advances in thoracic oncology continue to drive the development of innovative therapeutic strategies across lung cancer subtypes and stages.15–17 Clinical trials conducted by the Swiss Cancer Institute highlight the evolution of multimodal approaches integrating immunotherapy, chemotherapy and targeted agents. Key findings from the trials presented at ELCC 2025 and discussed in this article are summarized in Table 1. Other ongoing studies include SAKK 16/18 evaluating preoperative durvalumab combined with radiotherapy in locally advanced NSCLC18; the registry and study SAKK LuCa 2 designed to collect health-related data from patients with advanced EGFR-mutant NSCLC,19 the phase III Salvage trial comparing maintenance systemic therapy alone versus systemic therapy plus local ablative treatment in stage IV NSCLC,20 and the phase II study ETOP CHESS assessing the efficacy of radical immunotherapy and chemotherapy, stereotactic radiotherapy and surgery in oligometastatic NSCLC.21 Ongoing and future research will play a pivotal role in refining therapeutic approaches and individualizing care for patients with lung cancer.
Conflict of interest
Sacha I. Rothschild declared receiving consulting fees (payment to the institution) from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eisai, Eli Lilly, Merck Serono, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, PharmaMar, Roche Pharma, Roche Diagnostics, Sanofi Aventis and Takeda; research support from Amgen, Astra-Zeneca, Merck and Roche; honoraria for lectures and presentations (payment to the institution) from Amgen, AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme Oncology, Novartis, Roche Pharma, Roche Diagnostics and Takeda; payment for expert testimony (payment to the institution) from AstraZeneca, Bristol-Myers Squibb and Roche Pharma; support for travel and accommodations (payment to the institution) from Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Roche and Takeda; and reports participation on the Data Safety Monitoring Board (payment to the institution) of Roche and membership of the Federal Drug Commission of the Federal Office of Public Health. David König received institutional grants from Geistlich-Stucki Stiftung; consulting fees from AstraZeneca, Merck Sharp & Dohme und Novartis; honoraria for lectures, presentations or speakers’ bureaus from Amgen, Sanofi, Swiss Oncology in Motion and Mirati; travel support from Sanofi, Amgen and Roche; and holds positions on advisory boards from AstraZeneca, PharmaMar, Merck Bristol-Myers Squibb and Merck Sharp & Dohme. Laetitia A. Mauti declared receiving consulting fees from Sanofi, Daiichi Sankyo and Janssen; honoraria for lectures from Amgen and AstraZeneca; travel support from AstraZeneca, Roche, Sanofi, Amgen, Pharmamar and Pfizer; payment for expert testimony from BMS; participation on a Data Safety Monitoring Board or Advisory Board from Merck, Sanofi, Novartis, AstraZeneca, MSD, Pfizer, Regeneron, Roche and Janssen; and a leadership/fiduciary role with the Swiss Cancer Institute. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way. Other authors have declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
The authors have created and approved the final manuscript.

_and_progression-free_survival_(pfs)_at_a_me.png)

_and_progression-free_survival_(pfs)_at_a_me.png)