Introduction
Hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancer is the most prevalent molecular subtype, representing approximately 70% of all breast cancer diagnoses.1 While early-stage HR+/HER2− disease is potentially curable, its advanced metastatic stage remains incurable and presents a complex therapeutic challenge.2,3 In advanced disease, treatment is palliative, aiming to prolong survival, preserve functional status and quality of life and delay the initiation of cytotoxic chemotherapy.
For the majority of patients with HR+/HER2− metastatic breast cancer, endocrine therapy (ET) is the cornerstone of first-line management, except in cases of visceral crisis, life-threatening disease or documented endocrine resistance.4 The therapeutic armamentarium comprises selective estrogen receptor modulators (SERMs, such as tamoxifen), aromatase inhibitors (AIs, including letrozole, anastrozole and exemestane) and selective estrogen receptor degraders (SERDs, e.g., fulvestrant).
While the initial response to ET is often favorable, most patients eventually experience disease progression due to resistance, which remains the main barrier to durable disease control.2,5,6 Endocrine resistance arises from a complex interplay of genomic alterations and signaling pathway dysregulations, rather than being driven by a single underlying mechanism. Among the key contributors are mutations in the ESR1 gene causing ligand-independent estrogen receptor (ER) activation, PTEN loss resulting in aberrant phosphatidylinositol 3-kinase (PI3K)/AKT signaling and fibroblast growth factor receptor 1 (FGFR1) gene amplification promoting estrogen-independent tumor proliferation and survival. These molecular alterations provide a rationale for developing targeted therapies to overcome resistance and defer the need for chemotherapy.
Current treatment landscape in advanced HR+/HER2– breast cancer
Therapeutic strategies for HR+/HER2− advanced breast cancer have recently expanded significantly with the introduction of novel targeted agents tailored to the molecular profile of the disease. One of the major developments was the integration of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, which disrupt cell cycle progression and tumor growth.7 The addition of CDK4/6 inhibitors, including palbociclib, ribociclib and abemaciclib, to ET has significantly improved patient outcomes and is now considered the standard of care in the first-line setting for advance-stage HR+/HER2− disease.8
The phase II PALOMA-1 trial was the first pivotal proof-of-concept study to demonstrate that adding palbociclib to letrozole improves progression-free survival (PFS) in postmenopausal women with ER-positive (ER+)/HER2− advanced breast cancer.9,10 This benefit was confirmed in the phase III PALOMA-2 trial, which showed a significant PFS benefit, although no overall survival (OS) advantage with palbociclib was observed in the final analysis.11,12 Furthermore, ribociclib was evaluated in the phase III MONALEESA trial program. The MONALEESA-2 study demonstrated a significant PFS and OS benefit with ribociclib plus letrozole in treatment-naïve postmenopausal patients with HR+/HER2− advanced breast cancer.13–15 MONALEESA-3 demonstrated similar efficacy of ribociclib when combined with fulvestrant in men and postmenopausal women who had received ≤1 prior line of ET, showing consistent improvements in both PFS and OS.16–18 MONALEESA-7 extended these PFS and OS benefits to premenopausal women.19,20 Finally, in the phase III MONARCH-3 study, which included postmenopausal women with HR+/HER2− advanced breast cancer and no prior systemic therapy, abemaciclib combined with a nonsteroidal AI significantly improved PFS, while OS did not reach the prespecified threshold for statistical significance.21,22
In addition to CDK4/6 inhibitors, several targeted agents have been developed to address the molecular vulnerabilities in endocrine-resistant disease. For patients harboring germline BRCA1/2 mutations, poly (ADP-ribose) polymerase (PARP) inhibitors such as olaparib and talazoparib exploit deficiencies in homologous recombination repair, inducing synthetic lethality in tumor cells.23–25 The clinical utility of olaparib was established in the phase III OlympiAD trial, which demonstrated significantly improved objective response rate (ORR) and PFS versus physician’s choice of chemotherapy.26–28 Similarly, the phase III EMBRACA study confirmed the clinical activity of talazoparib, with significant improvements in PFS and patient-reported outcomes (PROs).29,30
Endocrine strategies have also evolved with the development of oral SERDs. Elacestrant, which was assessed in the phase III EMERALD trial, yielded improved PFS compared with standard ET in patients with ESR1-mutated tumors.31,32
Furthermore, antibody-drug conjugates (ADCs) have emerged as potent therapeutic options in later-line settings.33 Sacituzumab govitecan demonstrated clinical benefit in heavily pretreated HR+/HER2– patients in the TROPiCS-02 study.34 Trastuzumab deruxtecan, which was initially developed for HER2-positive disease, exhibited efficacy in HER2-low and HER2-ultralow breast cancer subtypes, as shown in the DESTINY-Breast04 trial.35,36
The PI3K/AKT/PTEN pathway and targeted inhibitors in HR+/HER2– breast cancer
Among the most frequently dysregulated signaling pathways implicated in endocrine resistance in HR+/HER2– breast cancer is the PI3K/AKT/PTEN axis.37–39 Abnormal activation of this pathway is observed in approximately 50% of HR+/HER2– breast cancers, either present at recurrence or as acquired resistance during therapies such as CDK4/6 inhibitors.
Several clinical trials have evaluated inhibitors that target this pathway, particularly in PIK3CA-mutated tumors. The PI3Kα-selective inhibitor alpelisib showed improved PFS and numerically better OS in the phase III SOLAR-1 trial in patients with endocrine-resistant disease following progression on AIs.40,41 More recently, the next-generation PI3Kα-selective inhibitor inavolisib, in combination with palbociclib and fulvestrant, significantly prolonged PFS compared with placebo plus palbociclib and fulvestrant in the phase III INAVO120 trial.42 This study included patients with PIK3CA-mutated, HR+/HER2– locally advanced or metastatic breast cancer who had relapsed during or within 12 months after the completion of adjuvant ET. Inavolisib is also being investigated in the phase III INAVO121 trial in patients following progression on CDK4/6 inhibitors and ET.43
Furthermore, the efficacy of PI3K/AKT/PTEN pathway inhibition in patients with HR+ advanced breast cancer was demonstrated using everolimus, an inhibitor of the mammalian target of rapamycin (mTOR), which functions as a key downstream pathway effector. In the BOLERO-2 study, everolimus improved treatment efficacy when combined with exemestane compared to endocrine therapy alone.44
Based on this growing body of evidence, the current guidelines of the European Society for Medical Oncology (ESMO)4,45 and the National Comprehensive Cancer Network (NCCN)46 recommend PIK3CA mutation testing in patients with HR+/HER2– recurrent, unresectable or stage IV breast cancer. For patients with confirmed PIK3CA mutations, fulvestrant and palbociclib combined with inavolisib are recommended in the first-line setting, whereas fulvestrant plus alpelisib remains the second-line option.
The guidelines also recognize the emerging role of AKT inhibitors, including capivasertib, particularly in tumors with PIK3CA, AKT1 or PTEN alterations.4,45,46 Capivasertib is a highly selective, orally bioavailable inhibitor of the serine/threonine kinase AKT that targets all three isoforms (AKT1/2/3) at nanomolar potency (Figure 1).47 Structurally, capivasertib is a pyrrolopyrimidine derivate that competitively binds to the catalytic domain of AKT in its active, phosphorylated conformation, disrupting downstream signaling through effectors such as mechanistic target of rapamycin complex 1 (mTORC1) and glycogen synthase kinase-3β (GSK3β).
In preclinical studies, capivasertib demonstrated robust suppression of AKT substrate phosphorylation in a concentration- and time-dependent manner across a range of tumor models, with antitumor effects being most pronounced in xenograft systems harboring oncogenic alterations in the PI3K/AKT pathway.47,48 Pharmacodynamic evaluations confirmed durable pathway inhibition, demonstrating sustained reductions in phosphorylated AKT (Ser473) and downstream effectors, such as p-S6 and p-4EBP1. Capivasertib has shown synergistic antitumor activity with endocrine and chemotherapeutic agents in vitro and in vivo, such as fulvestrant, docetaxel, lapatinib and trastuzumab, including in trastuzumab-resistant ER+ breast cancer models.47,48
The clinical potential of capivasertib was first demonstrated in the phase II FAKTION study, where the addition of capivasertib to fulvestrant significantly improved PFS, with a median of 10.3 months versus 4.8 months in the fulvestrant plus placebo arm (HR: 0.56 [95% CI: 0.38–0.81]; p=0.0023) in patients with AI-resistant advanced breast cancer.49,50 Treatment with capivasertib-containing therapy also led to prolonged median OS (29.3 months vs 23.4 months with placebo-containing therapy; HR: 0.66 [95% CI: 0.45–0.97]; p=0.035).
Building on these promising outcomes, the pivotal phase III CAPItello-291 trial was initiated to further evaluate the efficacy of capivasertib in endocrine-resistant breast cancer. Based on the findings of this trial, capivasertib in combination with fulvestrant was approved in Switzerland for the treatment of adult patients with locally advanced or metastatic HR+/HER2– breast cancer with one or more PIK3CA/AKT1/PTEN alterations following recurrence or progression on or after ET.51 The study design and key findings of the CAPItello-291 trial are discussed in detail in the following sections.
CAPItello-291: Study design
CAPItello-291 was a global, randomized, double-blind, placebo-controlled, phase III trial that was designed to evaluate the efficacy of capivasertib plus fulvestrant in patients with histologically confirmed HR+/HER2− inoperable locally advanced or metastatic breast cancer.52 This study enrolled adult premenopausal and postmenopausal women and men whose disease had relapsed or progressed during or within 12 months of prior AI therapy. Eligible patients were required to have measurable disease per RECIST v1.1 or at least one lytic or mixed lytic–blastic bone lesion with a soft-tissue component assessed by computed tomography (CT) or magnetic resonance imaging (MRI). Provision of tumor tissue for molecular profiling was mandatory, with genomic alterations determined by next-generation sequencing (NGS). Additional inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and receipt of ≤2 lines of prior ET and ≤1 line of chemotherapy for advanced disease. Prior treatment with a CDK4/6 inhibitor was permitted and mandated in at least 51% of the study population. Previous exposure to AKT, PI3K and mTOR inhibitors or SERDs was not allowed.
A total of 708 patients were randomized 1:1 to receive either capivasertib (400 mg orally twice daily on an intermittent schedule of 4 days on/3 days off) plus fulvestrant (500 mg intramuscularly every 14 days for the first three doses, then every 28 days) or placebo plus fulvestrant.52 Pre- and perimenopausal women additionally received a luteinizing hormone-releasing hormone (LHRH) agonist throughout the study period. Stratification factors included the presence of liver metastases, previous CDK4/6 inhibitor therapy and geographical region. The dual primary endpoint was PFS in the overall population and in the subgroup of patients with PIK3CA/AKT1/PTEN-altered tumors, as assessed by the investigator. The key secondary endpoints included OS, ORR, safety and PROs.
Adding capivasertib to fulvestrant improves PFS in patients with HR+/HER2– advanced breast cancer
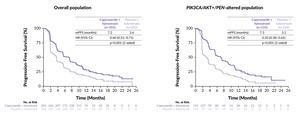
CAPItello-291 met its co-primary endpoints, demonstrating a statistically significant and clinically meaningful improvement in PFS with capivasertib plus fulvestrant compared with placebo plus fulvestrant, both in the overall population and in patients with PIK3CA/AKT1/PTEN-altered tumors, with a remarkable 50% reduction in the risk of disease progression or death observed in the pathway-altered subgroup (Figure 2).52
At a median follow-up of 13.0 months in the capivasertib plus fulvestrant arm and 12.7 months in the placebo plus fulvestrant arm, the median PFS in the overall population was 7.2 months versus 3.6 months, respectively (HR: 0.60 [95% CI: 0.51–0.71]; p<0.001).52 In the population with AKT pathway-altered tumors, the median PFS was 7.3 months with capivasertib versus 3.1 months with placebo (HR: 0.50 [95% CI: 0.38–0.65]; p<0.001).
An exploratory analysis in the subgroup of patients without PIK3CA, AKT1 or PTEN alterations (comprising 44% of the study population) yielded a PFS HR of 0.79, suggesting that the clinical benefit of capivasertib was predominantly driven by patients with genetically altered tumors.52 Therefore, the regulatory approval of the capivasertib plus fulvestrant combination by the European Medicines Agency (EMA) for patients with HR+/HER2− advanced breast cancer was granted based on the findings in the PIK3CA/AKT1/PTEN-altered subgroup.53
Notably, improved PFS with capivasertib plus fulvestrant was observed across all key clinically relevant subgroups including patients previously treated with CDK4/6 inhibitors and those with liver metastases.52
The OS data were immature at the time of the primary analysis. However, a favorable trend was observed with capivasertib plus fulvestrant versus placebo plus fulvestrant.52 The estimated 18-month OS rate was 73.9% versus 65.0% in the overall population (HR: 0.74 [95% CI: 0.56–0.98]) and 73.2% versus 62.9% in the AKT pathway-altered population (HR: 0.69 [95% CI: 0.45–1.05]), respectively. Ongoing follow-up will further assess OS in a pre-planned, event-driven interim analysis.
Capivasertib safety, tolerability and adverse event management
Capivasertib was generally well tolerated in the CAPItello-291 study, with a safety profile consistent with the known class effects of AKT inhibition.52 In the overall study population, any-grade adverse events (AEs) were reported in 96.6% of patients receiving capivasertib plus fulvestrant compared with 82.3% of those receiving placebo plus fulvestrant. Serious AEs occurred in 16.1% of patients in the capivasertib arm versus 8.0% of patients in the placebo arm, whereas serious AEs leading to death were observed in 1.1% and 0.3% of patients, respectively.
AEs were generally manageable through dose interruptions, reductions and discontinuations due to AEs, which occurred in 34.0%, 19.7% and 9.3% of patients receiving capivasertib-containing therapy, respectively.52 The safety profile of the combination was comparable across the overall and PI3K/AKT pathway-altered subpopulations. In the biomarker-altered cohort, the most frequently reported AEs with capivasertib versus placebo were diarrhea (any grade, 76.8% vs 18.8%; grade 3–4, 12.3% vs 0.8%), rash (any grade, 40.6% vs 8.3%; grade 3–4, 11.0% vs 0%) and nausea (any grade, 34.8% vs 13.5%; grade 3–4, 1.3% vs 0.8%).
Hyperglycemia associated with capivasertib treatment was predominantly low-grade and reported in 16.8% of patients receiving capivasertib plus fulvestrant compared with 3.8% of patients receiving placebo plus fulvestrant.52 Grade 3–4 hyperglycemia was infrequent, occurring in 1.9% of patients in the capivasertib arm and 0% in the placebo arm. These events typically emerged early in treatment, often during the initial treatment cycles and were rarely persistent or severe. Most cases were effectively managed with proactive blood glucose monitoring, temporary treatment interruptions and appropriate dose modifications, resulting in a low incidence of dose reductions or discontinuations due to hyperglycemia.
A range of supportive medications was employed to support the management of treatment-related AEs.52 These included loperamide for managing diarrhea, as well as topical and systemic corticosteroids and antihistamines for cutaneous toxicities. For hyperglycemia, glucose-lowering therapies such as metformin, insulin or other antihyperglycemic agents were used. This structured, multimodal approach to supportive care contributed to the overall tolerability of the regimen and facilitated continued treatment adherence in the majority of patients.
Conclusion
The CAPItello-291 trial established capivasertib as a clinically meaningful advancement in the management of endocrine-resistant HR+/HER2− advanced breast cancer, particularly for patients with tumors harboring PI3K/AKT/PTEN alterations. This oral, targeted AKT inhibitor demonstrated significant clinical benefit in delaying disease progression and offers a well-tolerated therapeutic option following progression on prior ET, irrespective of prior exposure to CDK4/6 inhibitors. The manageable safety profile, supported by structured AE management protocols, further enhances its integration into second-line treatment strategies in routine clinical practice.
The introduction of capivasertib into treatment guidelines for HR+/HER2− advanced breast cancer addresses a crucial unmet need in patients for whom therapeutic options are limited. Importantly, its targeted mechanism of action offers a more personalized treatment approach, supporting the growing role of molecular profiling in guiding individualized treatment strategies for advanced breast cancer. As OS data continue to mature and real-world experience with capivasertib accumulates, the positioning of capivasertib in the treatment landscape of HR+/HER2− advanced breast cancer is expected to become increasingly well-defined.
Taken together, this development not only expands the available treatment options but also underscores the shift toward biomarker-driven care, with the aim of optimizing outcomes in this challenging patient population with significant clinical need.
Conflict of interest
Marcus Vetter has received honoraria for advisory board roles from Pfizer-Seagen, Eli Lilly, Novartis, Roche, ExactScience, AstraZeneca, Gilead, Daiichi Sankyo, GSK and Stemline; research grants from Roche, GSK, ExactScience and Gilead/SAKK; travel support from Bayer, Boehringer Ingelheim, Amgen and Gilead; and holds company participations in ASC Oncology CH. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way. Other authors have declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
All authors contributed to and approved the final manuscript.