Introduction
Small cell lung cancer (SCLC), the most aggressive form of lung cancer, accounts for approximately 13 –15% of all primary lung cancer cases.1 Due to its high cell turnover rate, SCLC often shows a rapid response to chemotherapy and/or radiotherapy. However, in nearly all cases, recurrence is inevitable, leading to an overall poor prognosis.2 About 70% of patients are diagnosed with extensive-stage (ES) disease and can thus only be treated in a palliative approach.3 Platinum doublet chemotherapy, in combination with etoposide, was initially introduced in the 1980s and has since remained the standard first-line treatment for SCLC.4 Since then, immunotherapy targeting the programmed death-ligand 1 (PD-L1) axis has been developed and other novel therapeutic approaches are under investigation. Efforts have increasingly focused on understanding the role of different subtypes of SCLC and identifying relevant biomarkers, which may pave the way for new treatment options. Unlike in non-small cell lung cancer (NSCLC), PD-L1 expression and tumor mutational burden appear to have limited predictive value for the response to programmed cell death protein 1 (PD-1)/PD-L1-targeting therapies in SCLC.5 In contrast to NSCLC, a hallmark of SCLC is the absence of mutually exclusive, targetable oncogenic driver mutations.6,7 Currently, no therapeutic distinctions are made in the treatment of all SCLC patients. However, based on the patterns of transcription factor programs and immune pathway activation (ASCL1, NEUROD1 and POU2F3), four distinct SCLC subtypes have emerged: SCLC-A, SCLC-N, SCLC-P and SCLC-I. Among these, patients with the inflamed subtype (SCLC-I) achieve the greatest benefit from the addition of anti-PD-L1 therapy to chemotherapy.8 Novel targets, such as delta-like ligand 3 (DLL3), Trop-2, B7-H3, SLFN11 and even vascular endothelial growth factor (VEGF), are emerging as potentially significant players in future treatment strategies for SCLC. In this review, we discuss the latest study results and their implications for the future treatment of patients with SCLC, both in limited-stage (LS) and ES disease.
Immunotherapy consolidation for limited-stage disease: ADRIATIC trial
Until recently, the standard treatment for stage I-III disease comprised a combination of etoposide-platinum (EP) chemotherapy with concurrent thoracic radiotherapy, along with the consideration of prophylactic central nervous system (CNS) irradiation, followed by regular follow-up.5 In the past, consolidation immunotherapy was explored, e.g. in the context of the STIMULI phase II trial, where consolidation immunotherapy with nivolumab and ipilimumab after standard treatment for LS-SCLC did not improve progression-free survival (PFS) compared to observation.9 High rates of adverse events (AEs) and a short duration of treatment in the experimental group likely impacted the outcomes of the trial.
Furthermore, concurrent administration of atezolizumab to chemoradiation did not improve OS for patients with LS-SCLC in the second interim analysis of the NRG-LU005 trial.10
However, during the last year’s Annual Meeting of the American Society of Clinical Oncology (ASCO), data from the ADRIATIC study were presented for the first time. This is a randomized, double-blind, global, multicenter, phase III clinical study that investigated the efficacy and safety of the PD-L1 inhibitor durvalumab, placebo, or durvalumab in combination with the CTLA-4 inhibitor tremelimumab, as consolidation therapy (up to 24 months) for LS-SCLC patients without disease progression after concurrent platinum-based chemoradiotherapy.11 It was demonstrated that the addition of durvalumab (1,500 mg every four weeks for a duration of 24 months) following chemoradiotherapy resulted in a significantly longer overall survival (OS) compared to the placebo group, with median survival times of 55.9 months versus 33.4 months, respectively (HR: 0.73 [98.321% CI: 0.54–0.98]; p=0.01) (Figure 1). Furthermore, PFS was significantly longer in the experimental arm (median, 16.6 months [95% CI: 10.2–28.2] versus 9.2 months [95% CI: 7.4–12.9]). Superior OS and PFS for the durvalumab arm compared to the placebo arm were consistent across different subgroups, including stage, receipt of prophylactic cranial irradiation (PCI), frequency of radiotherapy, chemotherapy with cisplatin or carboplatin and timing of consolidation start. Importantly, there was no significant difference in the incidence of grade 3–4 toxicities between the two groups (24.4% for durvalumab vs 24.2% for placebo), although a slightly higher rate of grade 3–4 pneumonitis was observed, occurring in 3.1% of patients in the durvalumab group compared with 2.6% in the placebo group. Given these promising results, the incorporation of durvalumab treatment following chemoradiotherapy in patients with LS disease should be integrated into our daily clinical practice.
Immune checkpoint inhibitors for extensive-stage disease
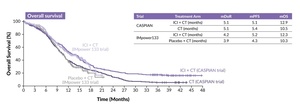
Checkpoint inhibition in ES disease: CASPIAN and IMpower133 trials
Since the introduction of PD-L1-directed immunotherapies and the demonstration of OS benefit of approximately two months with their addition to platinum-based chemotherapy, the standard treatment in ES disease has been supplemented either with durvalumab based on the results of the CASPIAN trial,12–14 or atezolizumab, as supported by the findings of the IMpower133 trial (Figure 2).15–17 Recently, 5-year OS data from IMpower133 were published showing that 12% of patients in the experimental arm were still alive.17 This finding confirms the durable effect of PD-L1 targeting in the setting of ES-SCLC, albeit unfortunately only for a minority of patients. Consequently, these therapies have been incorporated into current guidelines and clinical practice.5,18
Given the short duration of response (DoR) and a median OS of only 12.9 months in the CASPIAN trial and 12.3 months in the IMpower133 study, there remains a significant unmet medical need for improved treatment options in this patient population. Consequently, recent years have seen a focus on exploring biomarkers and developing novel therapeutic approaches to enhance existing treatment options.
Positive PFS and OS data for lurbinectedin and atezolizumab consolidation therapy: IMforte trial
Data from a phase III IMforte study comparing maintenance therapy with atezolizumab alone versus the combination of atezolizumab and lurbinectedin after four cycles of chemotherapy in ES-SCLC were recently presented at ASCO 202520 and simultaneously published in The Lancet.21 In this study, a total of 660 patients were randomized in a 1:1 ratio after four cycles of chemoimmunotherapy to receive either standard treatment with atezolizumab for maintenance, analogous to the standard of care in the IMpower133 trial, or atezolizumab in combination with lurbinectedin. The PFS was significantly longer in the lurbinectedin plus atezolizumab group compared to the atezolizumab group, with an HR of 0.54 (95% CI: 0.43–0.67]; p<0.0001). Similarly, OS was extended in the combination therapy group, with a stratified HR of 0.73 (95% CI: 0.57–0.95; p=0.017) (Figure 3).
Despite the statistically significant improvements in both PFS and OS favoring the combination therapy, it is important to highlight increased rates of AEs, especially hematological toxicity, associated with this treatment.21 Specifically, 38% of patients in the lurbinectedin plus atezolizumab group experienced grade 3–4 AEs, compared to 22% in the atezolizumab group (Tables 1 and 2).
Other checkpoint inhibitors: KEYNOTE-604, RATIONALE-312 and EXTENTORCH trials
In addition to the CASPIAN and IMforte trials, several other studies have investigated checkpoint inhibition in ES-SCLC. The randomized, double-blind, phase III KEYNOTE-604 study compared the PD-1 inhibitor pembrolizumab in combination with EP versus placebo plus EP in treatment-naïve ES-SCLC patients (n=453).22 PFS was significantly improved in the pembrolizumab arm (HR: 0.75 [95% CI: 0.61–0.91]; p=0.0023), with a 12-month estimated PFS rate of 13.6% versus 3.1% with placebo. Although there was an OS trend favoring pembrolizumab plus EP, the significance threshold was not met (HR: 0.80 [95% CI: 0.64–0.98]; p=0.0164). The long-term follow-up confirmed an ongoing clinically meaningful improvement in OS and PFS.23 At a median follow-up of 43.3 months, the median OS was 10.8 months in the pembrolizumab arm versus 9.7 months in the placebo arm (HR: 0.76 [95% CI: 0.53–0.93]) and 3-year OS rates were 15.5% versus 5.9%, respectively. The median PFS was 4.8 months in the experimental arm compared with 4.3 months in the control arm (HR: 0.70 [95% CI: 0.57–0.85]). The incidence of grade 3–5 AEs was 78.9% and 77.1% in the pembrolizumab and placebo arms, respectively.
Two other anti-PD-1 antibodies were investigated in phase III clinical trials conducted in China. RATIONALE-312 evaluated the efficacy and safety of tislelizumab plus chemotherapy as first-line treatment in patients with ES-SCLC (n=457).24 Adding tislelizumab to chemotherapy significantly improved OS (median, 15.5 months vs 13.5 months with placebo; HR: 0.75 [95% CI: 0.61–0.93]; p=0.0040) and PFS (median, 4.7 months vs 4.3 months with placebo; HR: 0.64 [95% CI: 0.52–0.78]; p<0.0001). Grade ≥3 treatment-related AEs (TRAEs) occurred in 86% of patients in each treatment arm and were mostly hematologic. The efficacy and safety of first-line toripalimab plus EP were evaluated in the EXTENTORCH study in 442 patients with ES-SCLC.25 Compared with placebo plus EP, toripalimab improved PFS (HR: 0.67 [95% CI: 0.54–0.82]; p<0.001) and OS, with a median OS of 14.6 months in the toripalimab arm versus 13.3 months in the placebo arm (HR: 0.80 [95% CI: 0.65–0.98]; p=0.03). The rates of grade ≥3 treatment-emergent adverse events (TEAEs) were similar between the two groups.
Real-world outcomes of chemoimmunotherapy in ES-SCLC
While randomized trials of chemoimmunotherapy in ES-SCLC have largely enrolled participants with a good performance status, a substantial proportion of patients encountered in routine clinical practice fall outside these criteria. Real-world studies have begun to address this gap by evaluating the effectiveness and safety of chemoimmunotherapy in broader and more heterogeneous patient populations. A retrospective, binational, multicenter study by Moliner et al. represents the largest reported real-world cohort of patients with ES-SCLC treated with first-line chemoimmunotherapy.26 The analysis included 436 consecutive patients across 10 institutions in the United Kingdom and 10 in Switzerland, all of whom received EP chemotherapy in combination with either atezolizumab or durvalumab. Notably, approximately one-third of the patients would not have met the eligibility criteria for the pivotal trials. The median PFS was 5.5 months and the median OS was 9.3 months, with a 2-year OS rate of 14%. TRAEs were reported in 51% of patients, and immune-related AEs occurred in 22% of patients. Importantly, survival outcomes and the incidence of AEs in this real-world setting were comparable to those reported in phase III trials, thus supporting chemoimmunotherapy as the standard first-line treatment for ES-SCLC in routine clinical practice.
What are the second-line treatment options?
As previously mentioned, most patients with ES disease experience recurrence within a median of less than six months following first-line therapy. In general, topotecan is considered a standard second-line chemotherapy, with an equivalent response rate and OS but better tolerability than the triplet combination of cyclophosphamide, adriamycin, and vincristine (CAV).27 Furthermore, lurbinectedin has become a new interesting second-line chemotherapy agent based on the promising response rate of 35.2% in a phase II clinical trial.28 Results from the confirmatory phase III LAGOON trial29 are awaited.
The current 2021 European Society for Medical Oncology (ESMO) guidelines recommend rechallenge with platinum-based chemotherapy for platinum-sensitive patients who have a treatment-free interval of three months or longer.5 This approach is supported by data from a randomized phase III trial, which demonstrated that patients with SCLC who received carboplatin plus etoposide had a median PFS of 4.7 months compared to 2.7 months with topotecan (HR: 0.57 [90% CI: 0.41–0.73]; p=0.0041).30 Patients with disease recurrence less than three months after completion of EP-based therapy are regarded as platinum-resistant and have a poor prognosis.31 For those patients, treatment options such as lurbinectedin, topotecan or best supportive care can be considered, depending on the patient’s overall condition and individual preferences.
Prophylactic cranial irradiation and consolidative thoracic radiotherapy in ES-SCLC
PCI has historically been used in ES-SCLC to decrease the risk of brain metastases, which are common in this population. The pivotal trial conducted by the European Organisation for Research and Treatment of Cancer (EORTC) demonstrated that PCI reduced the 1-year incidence of symptomatic brain metastases from 40% to 15% and was associated with an improvement in 1-year OS from 13.3% to 27.1%.32 However, the value of PCI has since been questioned by the Japanese phase III trial, which showed no survival benefit when patients underwent regular magnetic resonance imaging (MRI) surveillance and were treated with brain radiotherapy only upon progression.33 These findings have shifted clinical practice in some regions toward reserving PCI for select patients, particularly those with good performance status and no baseline brain metastases, while emphasizing MRI surveillance in others.
Consolidative thoracic radiotherapy (TRT) has also been investigated to improve local control in ES-SCLC. The randomized phase III CREST study demonstrated that adding 30 Gy in 10 fractions of TRT after chemotherapy significantly improved 2-year OS from 3% to 13%, although the primary endpoint of 1-year OS did not reach statistical significance (33% with TRT vs 28% without; p=0.066).34 Importantly, intrathoracic progression was substantially reduced (19% vs 46%). The multicenter, single-arm, open-label phase II SAKK 15/19 trial further explored this concept by combining TRT with durvalumab in the maintenance setting after first-line carboplatin and etoposide plus durvalumab.35 At the 24-month follow-up, adding TRT to chemoimmunotherapy resulted in a similar 12-month PFS rate versus control (16.5% vs 12.5%) and a favorable median OS of 15.8 months compared to historical controls without a significant increase in the incidence of clinically severe TRAEs.
New hope on the horizon for patients with SCLC: Emerging therapies
Currently, several new substances and therapeutic combinations are being investigated in SCLC, showing promising results in early studies. These will be elaborated on in more detail below.
The emerging role of new antibody-drug conjugates
Targeting B7-H3
IDeate-Lung01: Ifinatamab deruxtecan (I-DXd)
One such substance is the antibody-drug conjugate (ADC) ifinatamab deruxtecan (I-DXd), which targets B7-H3 (CD276), a type 1 transmembrane protein that belongs to the B7 family, which also includes PD-L1.36 In a study involving 107 patients with SCLC, B7-H3 was expressed at moderate-to-high levels in 69% of the participants. Patients with B7-H3-positive tumors exhibited shorter OS compared to those with B7-H3-negative tumors.36 I-DXd was evaluated in previously treated ES-SCLC at 8 mg/kg and 12 mg/kg doses as part of the phase II IDeate-Lung01 study.37,38 Among the participants, 28.4%, 50% and 21.6% had received one, two and three prior lines of therapy, respectively. Additionally, 51.1% of patients were chemotherapy-free for <90 days, while 46.6% were chemotherapy-free for ≥90 days.
The results indicated an objective response rate (ORR) of 26.1% for the 8 mg/kg dose in 46 patients, with a disease control rate (DCR) of 90.4%.39 In the 12 mg/kg dose cohort, the ORR was 54.8%, with a DCR of 90.5% (with a follow-up period of approximately 15 months). The treatment demonstrated rapid responses, with a time to response (TTR) of 1.4 months (Figure 4). PFS was 4.2 months for the 8 mg/kg dose and 5.5 months for the 12 mg/kg dose. The median OS was 9.4 months for the 8 mg dose and 11.8 months for the 12 mg dose. Notably, the DoR was longer in the 8 mg/kg group at 7.9 months compared to 4.2 months in the 12 mg/kg group. This difference might be due to a higher percentage of second-line responders in the 8 mg/kg group.
Moreover, the treatment showed promising CNS response rates. In a subgroup of 37 patients with baseline brain metastases, the CNS-confirmed ORR was 37.8%, increasing to 56.3% in a subset of 16 patients with baseline brain target lesions.40 The treatment was generally well-tolerated, with gastrointestinal and hematologic toxicities being the most common AEs. These encouraging results support the initiation of the phase III IDeate-Lung02 study (NCT06203210) which evaluates I-DXd versus treatment of physician’s choice in patients with relapsed SCLC.41
ARTEMIS-001
Another agent that also targets B7-H3 is the ADC GSK5764227. In the phase Ia/b study involving pretreated patients, promising results were observed.42 At a dose of 8 mg/kg (n=31), the ORR was 58.1%, while at 10 mg/kg (n=21) the ORR was 57.1%. A phase III study is planned to compare the efficacy and safety of this novel agent with topotecan chemotherapy in patients with relapsed SCLC.
Targeting Trop-2
TROPICS-03: Sacituzumab govitecan
Trop-2 is a transmembrane glycoprotein that plays a role in the regulation of cancer cell growth.43–45 High levels of Trop-2 expression are observed in the majority of solid tumors, including breast, lung and urothelial cancers. Sacituzumab govitecan (SG) is a Trop-2-directed ADC. In the TROPICS-03 study, SG was evaluated in previously treated patients with ES-SCLC.46 The study included 43 patients who had received no more than one prior platinum-based first-line therapy. The median follow-up was 12.3 months.
The results indicated an ORR of 41.9% (95% CI: 27.0–57.9), with a median DoR of 4.7 months (95% CI: 3.52–6.70).46 The median PFS was 4.4 months (95% CI: 3.81–6.11) and the median OS was 13.6 months (95% CI: 6.57–14.78). Patients who were platinum-sensitive exhibited better and longer responses compared to those who were platinum-resistant, with an ORR of 47.8% versus 35%, respectively.
AEs primarily included cytopenias and gastrointestinal toxicity.46 TEAEs leading to dose reduction occurred in 16 patients (37.2%), with neutropenia and diarrhea reported in 16.3% and 7.0% of patients, respectively. Importantly, none of the patients discontinued SG due to a TEAE.
Currently, a phase III EVOKE-SCLC-04 study comparing SG with topotecan as second-line therapy in ES-SCLC is being planned.47
Targeting DLL3 with tarlatamab
Another promising therapy involves targeting DLL3 with bispecific T-cell engager tarlatamab. Tarlatamab exerts its effects by simultaneously binding to DLL3 on cancer cells and CD3 on T cells, thereby facilitating T-cell-mediated lysis of the tumor cells through immune synapse formation and cytotoxic activation.48 DLL3, an inhibitory protein involved in Notch signaling, is expressed on cancer cells in approximately 85% to 96% of patients with SCLC. While DLL3 is typically localized intracellularly in healthy cells, it is frequently aberrantly expressed on the surface of SCLC cells.49–52
An update on the phase II DeLLphi-301 study was recently presented at the World Conference on Lung Cancer (WCLC) 2024.53 This study included previously treated patients who had received two or more prior lines of therapy. Approximately one-third of the patients had undergone three or more prior treatments, while the remaining two-thirds had received two prior therapies.
The results revealed an ORR of 40% and a DCR of 70%.53 The median DoR was 9.7 months (95% CI: 6.9 to not estimable [NE]), with a median PFS of 4.3 months (95% CI: 2.9–5.6) and a median OS of 15.2 months (95% CI: 10.8 to NE). Notably, 26 of the 100 patients achieved sustained disease control for at least 52 weeks (Figure 5).
Interestingly, there appeared to be no significant difference in OS between platinum-resistant and platinum-sensitive patients.53 Among patients with CNS involvement, tumor shrinkage of ≥30% was observed in 10 of 17 patients (59%), with an intracranial DCR of 94% (16 of 17 patients; 95% CI: 71.3–99.9).54 The median duration of intracranial disease control was NE, with a range of 2.6 to over 13.9 months.
In terms of safety, 53% of patients developed grade 1–2 cytokine release syndrome (CRS) and 32% experienced pyrexia.54 Other commonly reported side effects included fatigue and anorexia. CRS typically occurred early in the treatment course, specifically during cycles 1 or 2. Immune effector cell-associated neurotoxicity syndrome (ICANS) was observed in 15% of patients, primarily within the first six months of treatment.
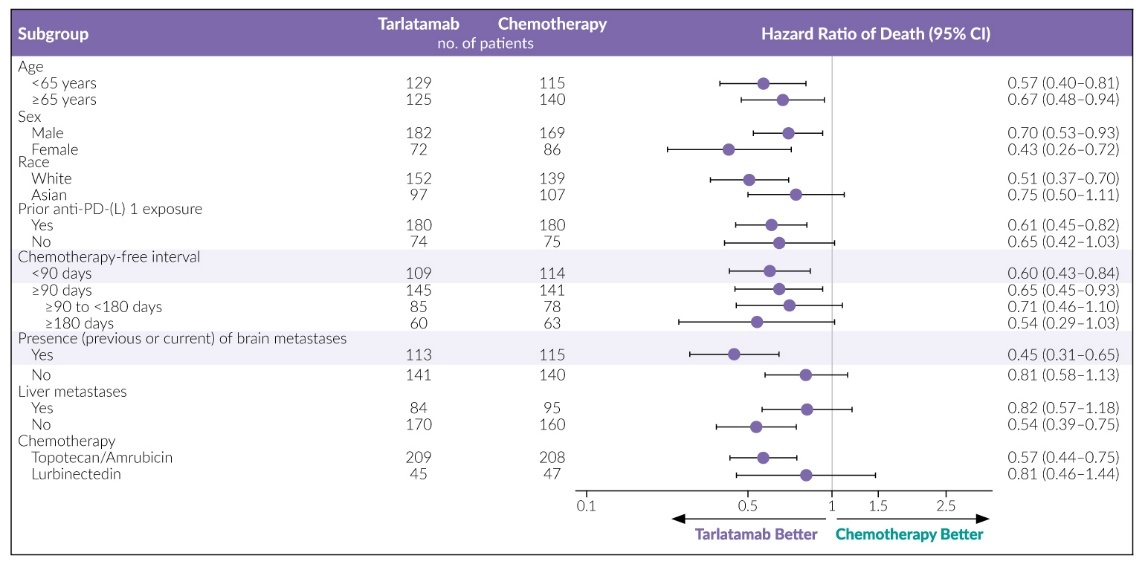
At ASCO 2025, the results from the phase III DeLLphi-304 study were presented.55,56 This trial compared tarlatamab to chemotherapy (topotecan, lurbinectedin or amrubicin) as a second-line treatment. Patients receiving tarlatamab (n=254) experienced a significantly longer OS than those receiving standard-of-care chemotherapy (n=255), with a median of 13.6 months (95% CI: 11.1 months to not reached) versus 8.3 months (95% CI: 7.0–10.2) (Figure 6). This survival benefit could be observed in all subgroups (Figure 7).
Additionally, tarlatamab demonstrated significant improvements in PFS and was more effective in alleviating cancer-related symptoms, such as shortness of breath and cough compared to chemotherapy. The rate of grade 3 or higher AEs was lower with tarlatamab (54%) than with chemotherapy (80%), and fewer patients stopped treatment due to AEs (5% vs 12%).
Another important study involving tarlatamab is DeLLphi-303, a phase Ib trial assessing the combination of tarlatamab with a PD-L1 inhibitor (either atezolizumab or durvalumab) as maintenance therapy for ES-SCLC following platinum-based first-line treatment.58 The study included a total of 88 patients. In terms of toxicity, 17% of patients receiving the tarlatamab plus atezolizumab combination experienced treatment interruptions and 4% discontinued therapy. Among those receiving tarlatamab plus durvalumab, 15% experienced treatment interruptions and 8% discontinued therapy. AEs were consistent with those associated with tarlatamab, and no unexpected toxicities were reported, indicating that the combination treatment was well tolerated.
Regarding efficacy, DCR was 62.5% (95% CI: 51.5–72.6), with a median DoR of 9.3 months (95% CI: 5.6 to NE).58 The OS rate at 9 months was 89% (95% CI: 78.7–94.3), indirectly compared to 60% for patients receiving atezolizumab as maintenance therapy alone in the IMpower133 study.59 Additionally, the median PFS with atezolizumab maintenance alone was 2.6 months, whereas the addition of tarlatamab extended it to 5.6 months. These findings suggest the possibility of a plateau in response among patients who benefit from tarlatamab, although a longer follow-up is needed to confirm these results.
Given its effectiveness as a treatment option in SCLC, tarlatamab is currently being further investigated in multiple ongoing DeLLphi trials (300-308) across various disease stages and lines of therapy, aiming to better define its future role in clinical practice.
ETER701: Additional VEGF targeting in first-line treatment using benmelstobart and anlotinib
The potential synergistic immunomodulatory effect of anti-VEGF and immune-oncology (IO) therapy has also been explored in SCLC. In a phase III study design, the oral VEGF multi-kinase inhibitor anlotinib and the anti-PD-L1 antibody benmelstobart were tested in combination with the standard carboplatin and etoposide chemotherapy regimen in patients with ES-SCLC.60
The results indicated a significant benefit for the VEGF plus IO combination.60 Specifically, the median OS for patients receiving benmelstobart and anlotinib in addition to carboplatin and etoposide was prolonged to 19.3 months, compared to 11.9 months for those receiving carboplatin and etoposide alone (HR: 0.61 [95% CI: 0.47–0.78]; p=0.0002) (Figure 8). However, the improvement in OS with anlotinib plus carboplatin and etoposide was not statistically significant, with a median OS of 13.3 months compared to 11.9 months (HR: 0.86 [95% CI: 0.67–1.10]; p=0.1723).
SLFN11 as a novel predictive and prognostic marker in SCLC
Another relevant biomarker currently being increasingly studied in SCLC is SLFN11. When overexpressed, SLFN11 inhibits tumor replication and growth by being rapidly recruited to stressed replication forks, where it induces replication arrest and contributes to tumor growth control.61 SLFN11 expression has been shown to be significantly associated with response to topoisomerase I and II inhibitors, alkylating agents and poly ADP-ribose polymerase (PARP) inhibitors across various cancer types, particularly in SCLC.
In the SWOG S1929 phase II study, atezolizumab combined with the PARP inhibitor talazoparib was investigated as a maintenance therapy in patients with ES-SCLC expressing SLFN11. The combination showed a PFS benefit compared to atezolizumab alone: PFS was significantly improved with the combination (HR: 0.70 [80% CI: 0.52–0.94]; p=0.056). The median PFS was 2.8 months (80% CI: 2.0–2.9) for atezolizumab alone and 4.2 months (80% CI: 2.8–4.7) for the combination. OS was not significantly different between the groups (HR: 1.17 [80% CI: 0.80–1.71]; p=0.30), with a median OS of 8.5 months (80% CI: 7.4–12.7) for atezolizumab alone and 9.4 months (80% CI: 8.1–14.2) for the combination.62
In the phase II ETOP 23-22 RAISE study, the addition of the PARP inhibitor niraparib to PD-L1 checkpoint blockade will be investigated as a first-line maintenance therapy for SCLC patients with high SLFN11 expression.63
Conclusions
Despite the latest insights and new studies, SCLC remains an aggressive disease with poor prognosis. However, after decades of stagnation, recent findings have sparked hope for improved treatment options. We eagerly anticipate the results of new phase III trials and their long-term follow-up data and look forward to the insights that future congresses will bring in the coming years.
Conflict of interest
Dr Sebastian Kraus serves as principal investigator for AstraZeneca and has received advisory board payments from Roche, Janssen, Servier and AstraZeneca, as well as travel grants from several pharmaceutical companies. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way.
Funding
The author has declared that no financial support has been received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_benefit_with_the_addition_of_programmed_death-ligand_1_(pd.jpeg)
_and_progression.jpeg)


_benefit_compared_to_standard-o.jpeg)

_benefit_of_vegf_targeting_(anlotinib)_and_pd-l1_blockade_(b.jpeg)
_benefit_with_the_addition_of_programmed_death-ligand_1_(pd.jpeg)

_and_progression.jpeg)


_benefit_compared_to_standard-o.jpeg)
_benefit_of_vegf_targeting_(anlotinib)_and_pd-l1_blockade_(b.jpeg)