Introduction
Urothelial carcinoma of the bladder is one of the most frequently diagnosed malignancies globally, with an estimated annual incidence of approximately 600,000 new cases and 200,000 related deaths.1 The primary etiological factor for bladder cancer is tobacco smoking, which is implicated in nearly 50% of all cases.2 Additionally, prolonged occupational exposure to carcinogenic substances, such as aromatic amines and polycyclic aromatic hydrocarbons, significantly contributes to disease risk. Approximately 75% of newly diagnosed bladder cancer cases present as non-muscle-invasive disease, characterized by a lower immediate mortality risk but a high propensity risk of recurrence and progression.3 Muscle-invasive bladder cancer (MIBC) is an aggressive form of bladder cancer with a high risk of recurrence and poor overall prognosis despite intensive local and systemic therapies.4 The current standard of care is cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy, which has been shown to improve disease control and survival outcomes.5 However, half of the patients are ineligible for cisplatin due to renal impairment or other comorbidities and thus there is a high unmet need for alternative treatment strategies.
Recent advancements in the treatment of metastatic urothelial cancer have initiated a paradigm shift in perioperative management, driven by the development of novel therapies and a better understanding of tumor biology.5–8 While neoadjuvant chemotherapy has been the mainstay for decades, ongoing research has aimed to improve current strategies and prolong patient survival by incorporating immunotherapy, targeted therapies such as antibody-drug conjugates (ADCs) and novel drug combinations into disease management. These agents provide potential benefits to patients with MIBC, particularly those ineligible for conventional chemotherapy. However, despite these advancements, challenges remain, including optimal patient selection, toxicity and durability of response.
Neoadjuvant chemotherapy as standard of care
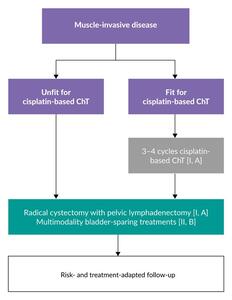
Cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy provides a 5% absolute overall survival (OS) benefit at five years compared with cystectomy alone in eligible patients with MIBC.9,10 The most commonly used neoadjuvant regimens, cisplatin plus gemcitabine (GC) and dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC), are recommended treatment options by the European Society for Medical Oncology (ESMO).6 Based on the ESMO guidelines, neoadjuvant chemotherapy is the preferred approach in these patients (Figure 1), while evidence supporting the use of adjuvant cisplatin-based chemotherapy in those who did not receive neoadjuvant therapy remains weak. Major guidelines from organizations such as the European Association of Urology (EAU),5 the National Comprehensive Cancer Network (NCCN)8 and the American Urological Association/Society of Urologic Oncology (AUA/SUO)11 generally align with these recommendations.
The benefits of neoadjuvant chemotherapy include early administration when the burden of micrometastatic disease is low, improved patient tolerance to chemotherapy and better compliance before cystectomy. Another key advantage is the ability to assess in vivo chemosensitivity through pathological response, particularly when achieving ypT0, ≤ypT1 or ypN0 and negative surgical margins. However, a potential drawback is that delayed cystectomy in patients who do not respond to chemotherapy may negatively impact overall oncological outcomes.
The recommendation for neoadjuvant chemotherapy in MIBC is based on data from several phase III clinical trials performed decades ago. This includes the SWOG-870 trial, which demonstrated that adding three cycles of MVAC to radical cystectomy improved OS in patients with MIBC (T2–T4a), with a median of 77 months versus 46 months with radical cystectomy alone and 5-year OS rates of 57% versus 43%, respectively (p=0.06).12 Furthermore, among MVAC-treated patients undergoing cystectomy (initial stage T2: 50% of the study population; initial stage T3–4a: 30%), 38% achieved complete pathological response (pCR; pT0), compared with 15% in the cystectomy-alone group (p<0.001). Further supporting the role of neoadjuvant chemotherapy, the BA0630894 study showed that three cycles of cisplatin, methotrexate and vinblastine (CMV) led to a statistically significant 16% reduction in the risk of death (p=0.037). This translated into an increase in 10-year OS from 30% to 36%, with a median OS improvement from 37 to 44 months.13
More recently, the phase III VESPER trial investigated the efficacy of ddMVAC (six cycles) compared with GC (total of four cycles) in patients with MIBC.14,15 While the study did not meet its primary endpoint of progression-free survival (PFS) in the overall population (3-year PFS rate: 64% vs 56%; HR: 0.77 [95% CI: 0.57–1.02]; p=0.066), significant benefits were observed in the neoadjuvant group comprising 88% of the study population.14 In this subset, the 3-year PFS rate was significantly higher in the ddMVAC arm, at 66% versus 56% in the GC arm (HR: 0.70 [95% CI: 0.51–0.96]; p=0.025). At five years, OS was not improved in the ITT population (64% vs 56%; stratified HR: 0.79 [95% CI: 0.59–1.05]).15 The underpowered analysis of OS in the neoadjuvant subgroup showed improvements in OS with ddMVAC compared with GC, with OS rates of 66% versus 57%, respectively (HR: 0.71 [95% CI: 0.52–0.97]; p=0.032) (Figure 2). Of note, based on these findings, dose-dense MVAC has become the preferred neoadjuvant regimen in the NCCN guidelines,8 whereas ESMO and EAU guidelines have not adaped their guidelines based on the lack of OS improvement in the ITT population.6
The pathological response is another important measure of treatment efficacy. In VESPER, pCR (ypT0pN0) was achieved in 42% of patients in the dose-dense MVAC group and 36% in the GC group (p=0.2).16 This result aligns with findings from other neoadjuvant trials, where pCR rates typically remain below 50%.17 While pCR is commonly used as a surrogate endpoint in clinical trials, its correlation with long-term outcomes such as metastasis-free survival (MFS) and OS remains under scrutiny.18 Neither individual-level nor trial-level surrogacy between pCR and time-to-event outcomes, such as event-free survival (EFS), disease-free survival (DFS) and OS, has been definitively established. However, pCR may still serve as a useful biomarker for guiding subsequent therapy, particularly in trials exploring treatment intensification for patients who do not achieve a complete pathological response mostly by adding on adjuvant treatment.
Optimizing strategies for neoadjuvant therapy in cisplatin-eligible patients
With the emergence of novel systemic therapies, optimizing neoadjuvant treatment for patients with MIBC requires prioritizing survival outcomes and identifying patients most likely to benefit. Expanding drug classes provide new treatment possibilities, including programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, with or without cytotoxic T lymphocyte-associated protein 4 (CTLA4) blockade, and ADCs. For cisplatin-eligible patients, integrating traditional chemotherapy with these newer agents in perioperative settings has shown promising results. Trials combining cisplatin-based chemotherapy with PD-1/PD-L1 inhibitors have demonstrated pCR rates ranging from 30% to 58%, particularly with agents such as durvalumab,19 nivolumab,20 pembrolizumab,21,22 atezolizumab23 avelumab24,25 and durvalumab combined with tremelimumab.26 Results from clinical trials on neoadjuvant, perioperative and adjuvant regimens in cisplatin-eligible patients with MIBC are summarized in Table 1.
Recent advances in perioperative strategies are highlighted by the phase III NIAGARA trial, which demonstrated significant improvements in EFS and OS with perioperative immunotherapy plus neoadjuvant chemotherapy as compared with neoadjuvant chemotherapy alone.28,32 In this study, cisplatin-eligible patients were randomized to receive either neoadjuvant durvalumab plus GC, followed by radical cystectomy and adjuvant durvalumab (n=533), or neoadjuvant GC followed by radical cystectomy alone (n=530). The study met its co-primary endpoint of EFS, with an estimated EFS rate at 24 months of 67.8% in the durvalumab group and 59.8% in the chemotherapy alone group (HR: 0.68 [95% CI: 0.56–0.82]; p<0.001) (Figure 3).28 The 24-month OS rate was also significantly improved with durvalumab plus GC (82.2% vs 75.2%; HR: 0.75 [95% CI: 0.59–0.93]; p=0.01). Durvalumab-combination therapy was associated with significantly improved co-primary endpoint of pCR, with rates at 37.3% compared with 27.5% with GC alone (odds ratio: 1.60 [95% CI: 1.23–2.08]; p=0.0005),28 with EFS and OS improved both in patients with and without pCR in an exploratory post-hoc analysis.27 Furthermore, the secondary endpoints of MFS and DFS were also prolonged at 24 months.27 The MFS rates were 75.1% versus 65.1% (HR: 0.67 [95% CI: 0.54–0.83]; p<0.001) and DFS rates were 89.2% versus 82.2% (HR: 0.69 [95% CI: 0.52–0.91]; p=0.008) with durvalumab plus GC versus GC alone, corresponding to 33% and 31% reduction in MFS and DFS event risk, respectively.
Durvalumab has been recently approved by the U.S Food and Drug Administration (FDA) in combination with cisplatin and gemcitabine based on the NIAGARA data.33 The NCCN guidelines implemented the recommendation,8 whereas the European EAU5 and ESMO6 guidelines have not yet been updated.
Perioperative chemo-immunotherapy after induction therapy with intravesical recombinant Bacillus Calmette Guérin (BCG) in patients with MIBC is currently being investigated in the multicenter, single-arm phase II SAKK 06/19 trial.34 This study enrolled patients with operable pT2 or cT2–T4a, cN0–1 disease without contraindication for cisplatin to receive intravesical BCG followed by atezolizumab and chemotherapy with GC followed by radical cystectomy and lymphadenectomy. Atezolizumab is continued after surgery for 13 cycles in case of residual muscle-invasive disease (≥ypT2) or positive lymph nodes (ypN+) only. pCR at cystectomy is the primary endpoint.
Perioperative chemoimmunotherapy is also investigated in the ongoing phase III KEYNOTE-866 trial which evaluates the efficacy and safety of perioperative pembrolizumab or placebo in combination with chemotherapy in cisplatin-eligible patients with MIBC.35,36 Approximately 870 patients are randomly assigned in a 1:1 ratio to receive GC in combination with either neoadjuvant pembrolizumab or placebo followed by radical cystectomy and pelvic lymph node dissection and adjuvant pembrolizumab or placebo. The primary endpoints are pCR and EFS in all patients and patients with PD-L1 combined positive score (CPS) ≥10. The secondary endpoints include OS, DFS, pathologic downstaging rate and safety. The primary completion of the study is estimated to be in June 2025.
Guiding perioperative systemic therapy with molecular testing
Optimizing treatment strategies requires distinguishing between biomarkers that indicate who benefits most from therapy and who needs it.37 These two concepts are frequently conflated but require separate biomarkers to provide precise treatment guidance. A patient must need treatment to benefit from it, but a biomarker indicating necessity does not necessarily confirm the benefit. In this context, it is important to differentiate between prognostic and predictive biomarkers. A prognostic biomarker, such as positive circulating tumor DNA (ctDNA), indicates a higher risk of recurrence and identifies patients who may need additional treatment, while a predictive biomarker suggests a higher likelihood of benefit from a particular therapy, such as a checkpoint inhibitor.
Defining the benefit of treatment is particularly challenging, as no biomarker so far, including molecular subtypes, DNA damage response, gene alterations or PD1 immunohistochemistry, has been fully established in urothelial cancer. So far, the most promising biomarker for identifying patients who need perioperative systemic therapy is molecular residual disease (MRD) testing using ctDNA. Multiple studies have demonstrated its correlation with clinical outcomes, with particularly strong, although exploratory, data from the phase III IMvigor10 trial.30,31
Although this study did not show a significant benefit of adjuvant atezolizumab versus observation in DFS or OS in the intention-to-treat population,30 further analyses indicated a significant clinical benefit in patients who were positive for ctDNA at the start of therapy (37% of the study population).31 In this subset, patients receiving atezolizumab versus observation had improved both DFS (median, 5.9 months vs 4.4 months; HR: 0.58 [95% CI: 0.43–0.79]; p=0.0024) and OS (median, 25.8 months vs 15.8 months; HR: 0.59 [95% CI: 0.41–0.86]), with no difference for patients who were negative for ctDNA (Figure 4). The rate of ctDNA clearance at week 6 was significantly higher in the atezolizumab arm than that in the observation arm (18% vs 4%; p=0.0204). Further stratification revealed that patients with detectable ctDNA and elevated PD-L1 expression in their primary tumor showed the most benefit from adjuvant therapy.38
Based on these findings, the phase III IMvigor011 trial was designed to assess the clinical utility of ctDNA testing in the adjuvant setting in order to identify patients who need treatment and are most likely to benefit.39,40 To this end, patients with high-risk MIBC positive for ctDNA after cystectomy were randomized to receive either atezolizumab or placebo, while ctDNA-negative patients entered surveillance.40 Recent surveillance data showed that with a median follow-up of 16.3 months, the DFS rates were 92% at 12 months and 88% at 18 months in ctDNA-negative patients. The 12-month and 18-month OS rates were 100% and 98%, respectively. These findings suggest that serial ctDNA testing may provide greater clinical utility as a risk stratification tool than single-landmark ctDNA testing. Furthermore, the data support the notion that patients with high-risk MIBC who maintain ctDNA-negative status after cystectomy may be spared from adjuvant treatment. Another ctDNA-guided adjuvant trial in the MIBC setting is the TOMBOLA trial, which aimed to investigate whether serial ctDNA testing can identify patients who benefit from early immunotherapy after cystectomy.41 In this ongoing non-randomized study in Denmark, patients undergoing neoadjuvant chemotherapy and radical cystectomy for non-metastatic MIBC (cT2-4a cN0-1cM0) are monitored postoperatively with serial tumor-informed ctDNA testing. Patients who become ctDNA-positive receive atezolizumab regardless of imaging findings, while ctDNA-negative patients receive immunotherapy only upon detection of metastases on imaging. Among ctDNA-negative patients, the recurrence-free and OS rates were 98.5% and 100%, respectively, 360 days after surgery. With regard to the primary endpoint of complete response rate to atezolizumab in patients who became ctDNA-positive after radical cystectomy, 55% of the 44 patients with ctDNA-positive status converted to ctDNA-negative status with no evidence of disease on computed tomography (CT) scanning. These preliminary results suggest that ctDNA status after surgery is highly prognostic and that adjuvant immunotherapy may clear MRD.
Nonetheless, while the findings are promising, broader clinical adoption hinges on several critical factors: assay standardization, turnaround time, cost-effectiveness, and long-term outcomes beyond current follow-up periods. Also, the interpretation of ctDNA dynamics in the context of tumor heterogeneity and treatment-induced changes remains an area requiring further investigation. Future trials should explore integrating ctDNA with other biomarkers (e.g., PD-L1, TMB) to refine patient stratification and personalize therapy further.
Addressing challenges for cisplatin-ineligible patients
Approximately 50% of patients with MIBC are ineligible for cisplatin and their outcomes remain poor.12 Furthermore, half of the patients experience recurrence within two years of radical cystectomy and the 5-year survival after radical cystectomy is around 50%.42,43 This high-risk population, often characterized by frailty, renal impairment and upper urinary tract disease leading to kidney loss, represents a great unmet medical need and alternative therapies are required. Modern platinum-free regimens have shown promising results, including PD-1/PD-L1 inhibitors, either alone or in combination with anti-CTLA4 therapies, and ADCs, such as enfortumab vedotin and sacituzumab govitecan. These therapies have demonstrated promising pCR rates of 30–40% and are being actively investigated in ongoing neoadjuvant trials. Key studies exploring anti-PD-1/PD-L1 therapies include ABACUS (atezolizumab),44,45 PURE-1 (pembrolizumab plus gemcitabine)46,47 and NABUCCO (nivolumab plus ipilimumab) (Table 2).48,49 In addition, ADC-based strategies are under evaluation in trials such as EV-103 (enfortumab vedotin)50,51 and SURE-01/02 (sacituzumab govitecan, either alone or in combination with pembrolizumab).52
Innovative treatment strategy for this challenging patient population is the intravesical drug delivery system TAR-200, which provides continuous localized gemcitabine release within the bladder.53 In the randomized phase II SunRISe-4, TAR-200 was evaluated in combination with cetrelimab, an anti-PD-L1 therapy, versus cetrelimab alone in patients with MIBC (cT2–T4a) scheduled for radical cystectomy who were either ineligible for or refused neoadjuvant chemotherapy. Interim analysis showed a centrally confirmed pCR rate of 42% with TAR-200 plus cetrelimab and 23% with cetrelimab alone, with a pathologic overall response (pOR) rate (≤ypT1N0) of 60% versus 36%, respectively (Figure 5). When stratified by clinical stage, patients with cT2 disease (n=40) achieved a pCR rate of 48% and a pOR rate of 68% with TAR-200 plus cetrelimab; these rates were 23% and 39%, respectively, in those with cT3–T4a disease (n=13). In patients with incomplete transurethral resection of bladder tumor (TURBT) (n=9), the pCR rate was 56% and the pOR rate was 67% with TAR-200 plus cetrelimab, compared with 39% and 59%, respectively, in those with complete TURBT (n=44).
Conclusion and future perspectives
Recent data suggest a shift in the standard of care for cisplatin-eligible MIBC patients. The combination of neoadjuvant durvalumab with GC, followed by adjuvant durvalumab, has emerged as the new standard based on the results from the NIAGARA trial.28,32
Despite significant advancements in the treatment of MIBC, several questions remain unanswered and the role of immunotherapy in the neoadjuvant and adjuvant settings continues to be an area of active investigation. Moreover, there is ongoing debate whether patients achieving pCR still require adjuvant therapy. One of the most urgent challenges is how to effectively integrate ctDNA as a tool for personalizing treatment strategies. The potential of ctDNA to guide therapeutic decisions and optimize patient outcomes is promising; however, further prospective, ctDNA-guided, adaptive clinical trials are required to establish its clinical utility.
Several phase III clinical trials are currently investigating perioperative immune checkpoint inhibitors, ADCs and other novel therapies for both cisplatin-eligible patients, including KEYNOTE-866,35,36 KEYNOTE-B15/EV-30454 and ENERGYZE,55 as well as cisplatin-ineligible patients, such as VOLGA29 and KEYNOTE-905/EV-303 (Table 3).35,56 Results from these studies are expected to provide valuable insights into the evolving landscape of MIBC treatment, with the potential for a more personalized and effective approach.
Conflict of interest
Ursula Vogl received advisory board and speaker honoraria from Astellas, Roche, Janssen, Sanofi, Bayer, Merck, MSD, BMS, Pfizer, Novartis AAA, SAKK, Silvio Grasso consulting, healthbook (all institutional), travel grants from Merck, Janssen, Astra Zeneca and honorary and advisory board payments from healthbook, Astellas and Merck (private). Fabio Turco received a travel grant from Bayer, honorary from healthbook (private), Silvio Grasso consulting (institutional), SAKK (institutional), Merck (institutional). These funding entities did not play a role in the development of the manuscript and did not influence its content in any way. Other authors have declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
All authors contributed to and approved the final manuscript.
_at_five_years_based_on_the_type_of_neoadjuvant_chemotherapy_.jpeg)

_and_overall_survival_(r.jpg)
_rates_and_pathologic_overall_response_(por)_rates_in_pa.jpg)

_at_five_years_based_on_the_type_of_neoadjuvant_chemotherapy_.jpeg)

_and_overall_survival_(r.jpg)
_rates_and_pathologic_overall_response_(por)_rates_in_pa.jpg)