Introduction
In Switzerland, prostate cancer is the most commonly diagnosed cancer in men, with over 7,800 new diagnoses each year.1 Localized prostate cancer is generally associated with a favorable prognosis, with a 5-year survival rate of 100%, whereas this rate drops to approximately 38% in the metastatic setting.2
Treatment selection for localized disease is guided by tumor characteristics, including prostate-specific antigen (PSA) level, Gleason/the International Society of Urological Pathology (ISUP) grade and T-stage.3,4 Curative-intent options include active surveillance (low-risk cases), radical prostatectomy (RP) and radiotherapy (RT) alone or in combination with androgen deprivation (ADT). For patients with metastatic hormone-sensitive prostate cancer (mHSPC), ADT remains the backbone of therapy and is generally combined with novel androgen receptor pathway inhibitors (ARPIs) with or without chemotherapy depending on disease volume and previous local therapy. Progression on androgen deprivation defines the transition to metastatic castration-resistant prostate cancer (mCRPC). Treatment selection at this stage is multifactorial and is mainly influenced by prior therapies administered in the hormone-sensitive setting, genomic profile, prostate-specific membrane antigen (PSMA) avidity in PSMA-positron emission tomography (PET) and patient characteristics, including performance status (PS), comorbidities, symptoms and extent of the disease.
In recent years, novel therapeutic developments have been integrated into clinical practice in the advanced prostate cancer setting including ARPIs, taxanes, PSMA-targeted radioligand therapy (RLT) and poly (ADP-ribose) polymerase (PARP) inhibitors.4 Specifically, lutetium-177 [177Lu]-PSMA-617 has emerged as an effective and well-tolerated treatment approach for patients with pretreated mCRPC based on the VISION and TheraP trials. With PSMA expression reported to increase on enzalutamide in preclinical studies, the efficacy of this combination regimen has been explored in the ENZA-p trial as a first-line treatment in patients with mCRPC.5–8 PARP inhibitors have been investigated in the frontline mCRPC setting also extending beyond tumors with homologous recombination repair (HRR) loss.9 This article summarizes the most recent advancements in the treatment of prostate cancer presented at the 2025 ASCO Genitourinary (GU) Cancers Symposium and explores the challenges associated with integrating these findings into clinical practice.
High-risk localized prostate cancer: Scalpel or beam?
Recommended treatment options for high-risk localized prostate cancer, defined by either PSA >20 mg/mL, ISUP grade group 4–5 (Gleason score of 8–10) or clinical stage ≥T3, include external beam RT in combination with ADT for 18–36 months.3 RP with selective use of postoperative RT with or without ADT is a commonly used alternative. At ASCO GU 2025, results from an emulated randomized comparison, a statistical approach that mimics a randomized trial by emulating its conditions across separate datasets, were presented based on individual patient data (IPD) from two phase III randomized clinical trials, CALGB 90203 and NRG/RTOG.10
In the CALGB 90203 trial, 788 men with localized, high-risk prostate cancer were randomized 1:1 to receive either RP alone or in combination with neoadjuvant docetaxel (six cycles) and ADT.11 Notably, patients with positive surgical margins could receive adjuvant RT within six months of RP. The NRG/RTOG 0521 trial evaluated RT plus 24 months of ADT, with or without docetaxel (six cycles), in 612 patients with high-risk localized prostate cancer.12 The primary endpoint of the present analysis was to compare the cumulative incidence of distant metastasis between the treatment groups, accounting for death as a competing event.10
The analysis included 733 patients in the RP cohort, 366 who received RP plus neoadjuvant therapy and 367 who underwent surgery alone, and 557 patients in the RT cohort, including 279 patients treated with RT plus ADT and docetaxel and 278 treated with RT plus ADT alone.10 At baseline, patients receiving RT had a more unfavorable prognosis than those undergoing RP, including more advanced age (>70 years; 31% vs 9%; p<0.001), a higher grade (ISUP group 4–5 or Gleason score 8–10; 87% vs 84%; p=0.030), a higher tumor stage (T3–T4; 27% vs 17%; p<0.001) and higher PSA levels (>20 ng/mL; 43% vs 25%, p<0.001).
In the overall population, the 8-year incidence of distant metastasis (DM) was 23% in the surgery cohort and 16% in the RT cohort (subdistribution [s] HR: 0.56 [95% CI: 0.38–0.81]; p=0.002) (Figure 1).10 However, no significant differences were observed in the risk of death after progression (sHR: 0.79 [95% CI: 0.46–1.36]) or death after distant metastasis (sHR: 0.99 [95% CI: 0.58–1.71]). There was a significant difference in DM occurrence in favor of RT plus ADT independent of the use of additional chemotherapy in the respective subgroup analyses. This significance was, however, lost if patients undergoing RP received more intense additional therapy including ADT, docetaxel and personalized RT (triplet of quadruplet) compared to RT plus ADT (doublet) (sHR: 0.84 [95% CI: 0.51–1.37]; p=0.48).
In conclusion, an RT-based treatment regimen generally resulted in a lower incidence of distant metastasis than an RP-based approach for patients enrolled in these two phase III clinical trials.10 While RP combined with neoadjuvant therapy and selective postoperative RT showed similar outcomes to RT plus long-term ADT, these results raise the question of whether prostatectomy is an optimal choice for patients with high-risk prostate cancer, given that additional treatment may be required to achieve similar results.
Metastasis-directed therapy (MDT): Emerging treatment strategy for patients with oligometastatic prostate cancer
The evidence supporting metastasis-directed therapy (MDT) remains limited to a few small randomized phase II trials with inconclusive subgroup analyses.13 To address this gap, the WOLVERINE meta-analysis was conducted to congregate existing randomized data using MDT to provide a better understanding of treatment outcomes for patients with oligometastatic solid tumors including prostate cancer, defined as fewer than five metastases.
This study pooled individual patient data (IPD) from five randomized trials, including ARTO, EXTEND, ORIOLE, SABR-COMET and STOMP.13 Key clinical endpoints were harmonized across studies, including progression-free survival (PFS), radiographic PFS (rPFS), castration resistance-free survival (CRFS) (in the subset of patients with castration-sensitive disease only) and overall survival (OS). A total of 472 patients with oligometastatic disease were randomized in a 1:1 ratio to receive either MDT plus standard of care (SoC) (n=248) or SoC alone (n=224). Baseline characteristics were not well balanced: CRPC was diagnosed in 38% of patients in the MDT plus SoC group and 46% in the SoC alone group. Prior exposure to second-generation androgen receptor pathway inhibitors (ARPI) was reported in 50% of MDT versus 60% of SoC patients, respectively. As the inclusion criteria varied between trials, baseline imaging also differed, with 44% of patients in the MDT group and 35% in the SoC group having conventional imaging, while the remaining patients underwent PET-computed tomography (CT).
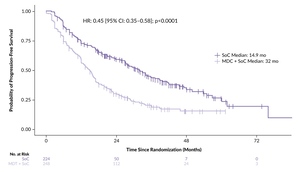
With a median follow-up of 41.0 months, the addition of MDT to SoC significantly improved PFS compared with SoC alone, with a median PFS of 32.0 months compared with 14.9 months with SoC alone (HR: 0.45 [95% CI: 0.35–0.58]; p<0.0001) (Figure 2).13 These benefits with MDT were consistent across all subgroups, including patients with castration-sensitive disease (HR: 0.51) and castration-resistant disease (HR: 0.45). MDT also demonstrated significant improvements in rPFS (HR: 0.59 [95% CI: 0.46–0.76]; p<0.001) and CRFS (HR: 0.58 [95% CI: 0.37–0.91]; p=0.020). A strong trend toward improved OS was observed with the addition of MDT (HR: 0.64 [95% CI: 0.40–1.01]; p=0.06), with 48-month OS rates of 87% in the MDT plus SoC group versus 75% in the SoC alone group.
The clinical benefit of MDT in addition to therapy with ADT and enzalutamide for oligometastatic CRPC was assessed in the phase II GROUQ-PCS 9 study.14 This trial enrolled patients with ADT-only pretreated mCRPC and 1–5 metastases who were randomized in a 1:1 ratio to receive either MDT with stereotactic body radiotherapy (SBRT) in addition to enzalutamide/ADT (n=52) or enzalutamide/ADT alone (n=48). Initially planned as an adaptive phase II/III trial across 13 sites, the trial was stopped early after enrolling 100 patients, as prior ARPI treatment was an exclusion criterion, but later became the SoC for patients with mHSPC severely affecting enrollment. The primary endpoint was rPFS by investigator assessment.
At baseline, the majority of patients had low-volume disease, defined as fewer than four metastases (84% in the SBRT arm vs 90% in the control arm) and Eastern Cooperative Oncology Group (ECOG) performance status of 0 (82% vs 67%).14 Notably, 50% of patients in the SBRT arm and 62% of patients in the control arm had tumors with an ISUP grade group 4–5 (Gleason score ≥8).
Results showed that the addition of SBRT to enzalutamide/ADT significantly improved rPFS, with a median rPFS of 4.6 years versus 2.3 years, respectively (HR: 0.48 [95% CI: 0.27–0.86]; p=0.014). The SBRT-containing regimen also reduced the risk of biochemical progression or death by 42% (HR: 0.58 [95% CI: 0.32–1.03]; p=0.07) and delayed the next line of therapy by a median of 2.2 years (5.1 years vs 2.9 years; HR: 0.42 [95% CI: 0.22–0.80]; p=0.009). There was no statistically significant difference in the risk of death with SBRT (HR: 0.71 [95% CI: 0.31–1.59]; p=0.41).
In conclusion, both the WOLVERINE meta-analysis and the phase II GROUQ-PCS 9 trial demonstrated improved clinical outcomes in patients with oligometastatic prostate cancer when MDT was added to the standard systemic therapy. Results from randomized phase III trials for MDT are, however, still limited. The analyses presented at ASCO GU 2025 supplement the data from the phase III STAMPEDE trial that demonstrated an OS benefit of RT in patients with newly diagnosed metastatic prostate cancer and a low metastatic burden, supporting the rationale for MDT in selected patient subgroups.15
Integration of novel agents to ARPI-based therapy in mCRPC
ENZA-p: 177Lu-PSMA-617 plus enzalutamide prolongs PFS in mCRPC
The ENZA-p (ANZUP 1901) trial was an open-label, randomized, phase II study that evaluated the efficacy and safety of adding lutetium-177 [177Lu]- PSMA-617 to enzalutamide in patients with mCRPC.16 Eligible patients had gallium-68 [68Ga]-PSMA PET-avid disease, no prior treatment with docetaxel or ARPI for mCRPC, and had at least two of the following risk factors for early enzalutamide failure: lactate dehydrogenase (LDH) or alkaline phosphatase (ALP) equal to or greater upper limit of normal, albumin <35 g/L, de novo metastatic disease at diagnosis, <3 years since initial diagnosis, >5 bone metastases, presence of visceral metastases, PSA doubling time of <84 days or prior abiraterone. Patients were randomized in a 1:1 ratio to receive either adaptive-dosed 177Lu-PSMA-617, administered in two to four 7.5 GBq doses every 6–8 weeks combined with 160 mg daily enzalutamide (n=83) or enzalutamide alone (n=79). The primary endpoint was PSA PFS, with OS and rPFS as the key secondary endpoints.
The baseline patient characteristics were well balanced between the two arms.16 Approximately 60% of patients had >20 PSMA-avid metastases and 52–58% presented with de novo metastatic disease at diagnosis. Half of the patients had previously received docetaxel, while only 11–14% had been treated with prior abiraterone for mHSPC and none had received other ARPIs in the mHSPC setting.
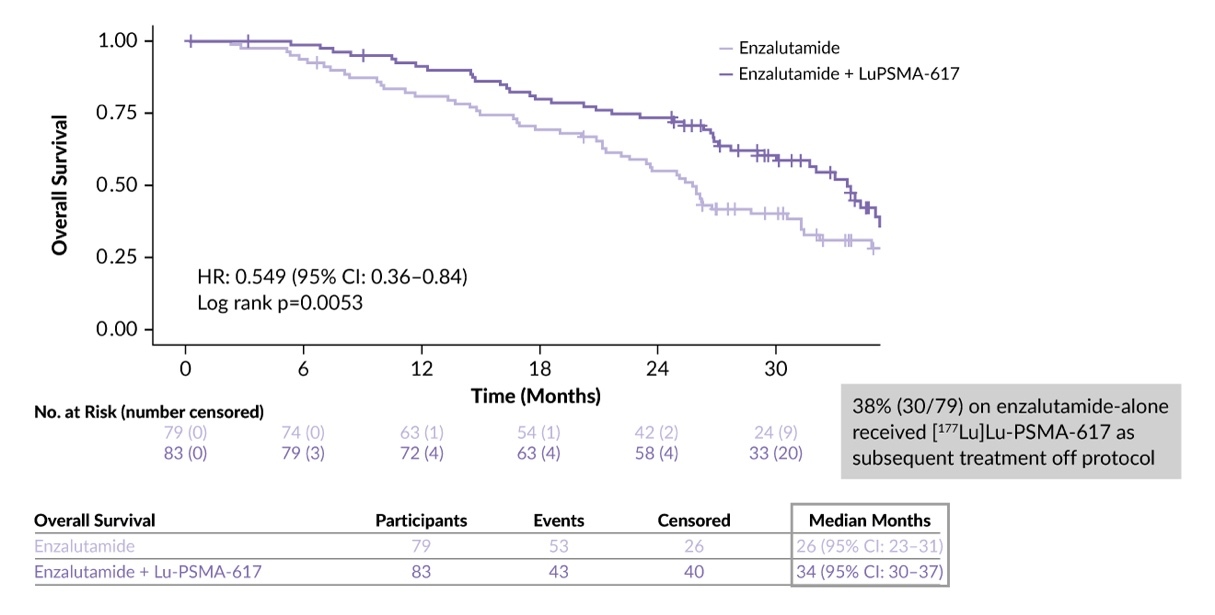
The addition of 177Lu-PSMA-617 to enzalutamide resulted in clinically meaningful improvements in PSA PFS (HR: 0.40 [95% CI: 0.28–0.59]; p=0.000001) and rPFS (HR: 0.61 [95% CI: 0.42–0.87]) compared with enzalutamide alone.8 A significant OS benefit for the combination therapy was found with a median OS of 34.0 months versus 26.0 months with enzalutamide alone (HR: 0.55 [95% CI: 0.36–0.84]; p=0.0053) (Figure 3).16,17 Of note, a higher proportion of patients in the control arm received subsequent therapies (73%) compared to the combination arm (58%). In the control arm, 38% of patients received 177Lu-PSMA-617 off protocol; this relatively low rate of post-progression 177Lu-PSMA-617 access may limit the ability to fully assess the treatment outcomes. Furthermore, the proportion of patients in the enzalutamide-alone arm who received subsequent PARP inhibitor was very low at only 3.7%, which may have impacted OS.
The findings from the ENZA-p study support the hypothesis that enzalutamide-induced PSMA upregulation has the potential for synergistic effect when co-targeting with 177Lu-PSMA-617.17 However, the mCRPC treatment landscape has evolved, particularly the current standard of ARPI use in mHSPC poses challenges for interpreting the ENZA-p data in the context of present-day clinical practice.
TALAPRO-2: Adding talazoparib to enzalutamide significantly improves clinical outcomes in patients with mCRPC
Co-inhibition of the PARP DNA repair mechanism (PARPi) and androgen receptor pathway (ARPI) has demonstrated positive results in treatment-naïve patients with mCRPC. The phase III randomized double-blind TALAPRO-2 trial evaluated the efficacy and safety of talazoparib in combination with enzalutamide versus enzalutamide alone as first-line treatment for patients with mCRPC.18 In the planned primary analysis at a median follow-up of 24.0 months, the median rPFS was not reached for talazoparib plus enzalutamide compared with 21.9 months for placebo plus enzalutamide (HR: 0.63 [95% CI: 0.51–0.78]; p<0.0001).18 At ASCO GU 2025 the final OS data for patients with and without HRR alterations were presented.9,19
In cohort 1, a total of 805 patients were prospectively assessed for HRR gene alterations in tumor tissue and randomized in a 1:1 ratio to receive enzalutamide with either talazoparib (n=402) or placebo (n=403).18 Cohort 2 included 399 patients with confirmed HRR gene alterations, comprising 169 patients from cohort 1 and 230 additional patients. The primary endpoint in both cohorts was rPFS and the key secondary endpoint was OS.
At a median follow-up of 47.0 months in cohort 1, talazoparib plus enzalutamide continued to demonstrate a significant rPFS benefit over enzalutamide alone, with a median rPFS of 33.1 months compared with 19.5 months, respectively [HR: 0.67 [95% CI: 0.551–0.807]; p<0.0001).9 Significant improvements in OS in the entire cohort were observed with talazoparib plus enzalutamide (HR: 0.8 [95% CI: 0.661–0.958]; p=0.0155), with a median of 45.8 months versus 37.0 months with enzalutamide alone (Figure 4). Post hoc subgroup analysis demonstrated a numerical improvement of OS in patients without BRCA mutations (n=439; HR: 0.75 [95% CI: 0.58–0.96]; nominal p=0.024) and those without any HRR gene alterations (n=314; HR: 0.78 [95% CI: 0.58–1.05]; p=0.10); however, this was not statistically significant.
In cohort 2, with a median follow-up of 44.2 months, the combination of talazoparib and enzalutamide significantly prolonged OS in patients with HRR-deficient mCRPC (HR: 0.62 [95% CI: 0.48–0.81]; p=0.0005), with a median OS of 45.1 months versus 31.1 months with enzalutamide alone.19 Among patients with BRCA 1/2 alterations (n=155), the combination therapy reduced the risk of death by 50% (HR: 0.50 [95% CI: 0.32–0.78]; p=0.0017), with a median OS not reached in the talazoparib plus enzalutamide arm versus 28.5 months in the enzalutamide alone arm. In the subgroup of patients with non-BRCA 1/2 HRR gene alterations, a numerical OS benefit was observed but this did not reach statistical significance (42.4 months vs 32.6 months, HR: 0.73 [95% CI: 0.52–1.02]; p=0.07).
In terms of safety, anemia was the most common treatment-emergent adverse event (TEAE) in patients treated with talazoparib plus enzalutamide, consistent with previous reports.9,19,20 Any-grade anemia was reported in approximately 67% of patients, with grade 3–4 events observed in up to 49% of patients.9,19 Anemia was manageable with dose modifications and/or standard supportive care, including blood transfusions, which were required in 42% of patients. The most frequent TEAEs leading to talazoparib dose reduction were anemia (45%), neutropenia (16%) and thrombocytopenia (6%).
In conclusion, findings from the TALAPRO-2 trial support the use of talazoparib plus enzalutamide as a first-line treatment option for patients with HRR gene-mutated mCRPC, particularly those harboring BRCA1/2 alterations. With this regimen, careful monitoring and management of hematologic toxicity are essential. As in ENZA-p, the interpretation and clinical applicability of the data are affected by the fact that the current treatment landscape in metastatic prostate cancer has changed since the vast majority received ADT plus APRI in the setting of mHSPC and this patient population is not represented in TALAPRO-2. The results cannot be extrapolated and the applicability of this approach in Switzerland remains limited. However, based on these promising results the implementation of PARPi in the setting of mHSPC is currently under investigation and these results might in the future indeed lead to changes in the treatment sequencing and clinical outcome.
Which subgroups of patients with mHSPC benefit more from ARPI therapy?
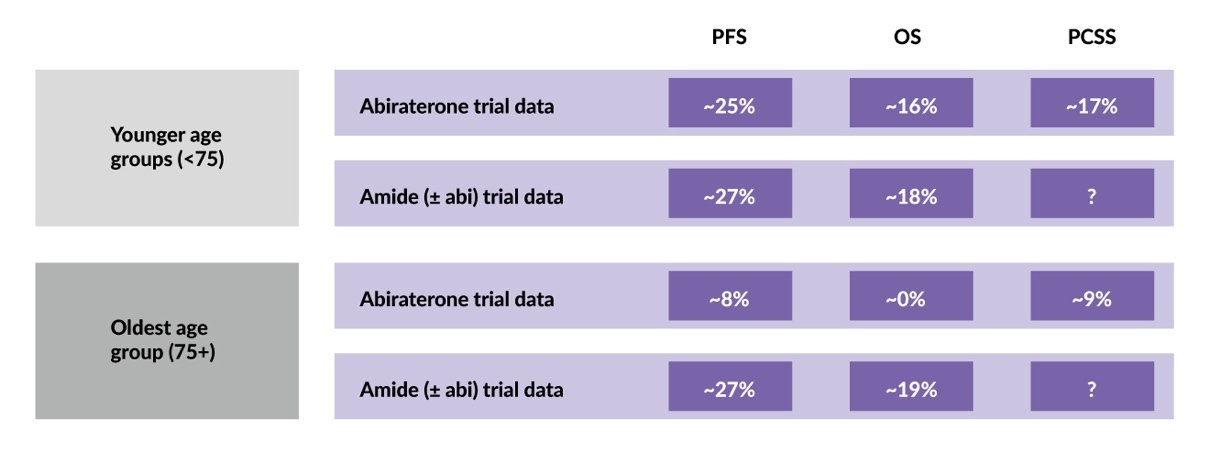
The clinical characteristics of patients with mHSPC may affect the outcome of ARPI treatment. STOPCAP is the first meta-analysis of IPD from ARPI trials that evaluated the efficacy of three ARPIs (enzalutamide, apalutamide and abiraterone) in men with mHSPC, aiming to assess differences between ARPI agents and whether treatment effects vary based on patient or disease characteristics.21 The analysis included data from 7,778 patients across seven clinical trials. Four trials investigated androgen biosynthesis inhibitors: STAMPEDE, LATITUDE and PEACE-1 using abiraterone and SWOG-1216 using orteronel. Three trials investigated “amides” with or without abiraterone: ENZAMET using enzalutamide, TITAN using apalutamide and STAMPEDE using a combination of abiraterone and enzalutamide.
The study demonstrated a clear benefit of ARPIs on OS (HR: 0.66 [95% CI: 0.62–0.71]) and PFS (HR: 0.51 [95% CI: 0.48–0.55]) in the majority of patients, with no difference observed between ARPI agents.21 For patients aged <75 years, a significant benefit was reported with the addition of any of the three ARPIs to ADT (Figure 5). In patients aged ≥75 years, enzalutamide and apalutamide continued to show survival benefits, whereas abiraterone reduced prostate cancer-specific mortality, but did not significantly improve the 5-year OS. No other patient or disease characteristics significantly modified treatment effects, including the volume or location of metastasis, clinical T-stage, Gleason sum score, nodal involvement or WHO performance status. These results highlight the need to consider the benefit/risk ratio of abiraterone and “amide” use in older patients with mHSPC.
Conclusions
-
In high-risk localized prostate cancer, RT combined with ADT appears to be the preferred treatment approach. RP remains an option and is often incorporated as a part of a multimodal treatment strategy.
-
For patients with oligometastatic prostate cancer, adding MDT to SoC was associated with favorable outcomes in both a meta-analysis and randomized phase II trial, supporting further investigation of this approach in large clinical trials.
-
In mCRPC, combining enzalutamide with either radioligand therapy (177Lu-PSMA-617), or a PARP inhibitor demonstrated superior OS compared with enzalutamide alone in patients not pretreated with ARPI. In the case of talazoparib, this benefit was only observed in patients with HRR alterations. However, the rapid adoption of ARPI as SoC in mHSPC complicates clinical trial data interpretation and highlights the need for investigation of earlier integration of these novel approaches.
-
In an IPD meta-analysis, younger patients with mHSPC benefited equally from enzalutamide, apalutamide and abiraterone added to ADT, whereas in those aged ≥75 years, only enzalutamide and apalutamide improved OS, suggesting the need to consider the benefit/risk profile of different ARPI classes in older patients.
Conflict of interest
The author has declared that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_with_surgery_versus_radiotherapy_in_the_ov.jpeg)

_for_talazoparib_(tala)_plus_enzalutamide_(enza)_ver.jpg)

_with_surgery_versus_radiotherapy_in_the_ov.jpeg)
_for_talazoparib_(tala)_plus_enzalutamide_(enza)_ver.jpg)