EMBER-3: Imlunestrant for ER+/HER2- advanced breast cancer
The EMBER-3 trial (NCT04975308) is a phase III, global, randomized, open-label study evaluating the efficacy and safety of imlunestrant in patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer.1,2 Injectable selective estrogen receptor degraders (SERDs), such as fulvestrant, have shown efficacy in certain settings but come with notable drawbacks, including the need for in-clinic administration and injection-site pain,3 thus burdening patients and reducing treatment adherence.4,5 Imlunestrant, an oral SERD, represents an innovative approach, offering a more patient-friendly alternative aimed at enhancing convenience, overcoming resistance to endocrine therapy (ET) and improving clinical outcomes, especially for patients carrying ESR1 mutations or with resistance to prior therapies.1,2
The study enrolled 874 adult patients with advanced ER+/HER2- breast cancer who experienced disease recurrence on or within 12 months of completing adjuvant treatment with an aromatase inhibitor, with or without a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor, or whose disease progressed after first-line treatment with an aromatase inhibitor, with or without a CDK4/6 inhibitor.1,2 Patients were randomized in a 1:1:1 ratio into three treatment arms to receive either imlunestrant monotherapy (n=331), standard-of-care (SoC) ET (n=330), which included fulvestrant or exemestane, or imlunestrant in combination with abemaciclib (n=213). Stratification factors included prior use of CDK4/6 inhibitors, the presence of visceral metastases and geographic region. The primary endpoints were investigator-assessed progression-free survival (PFS) for imlunestrant monotherapy versus ET in the ESR1-mutated and overall populations, as well as PFS for imlunestrant plus abemaciclib versus imlunestrant alone in the overall population. Key secondary endpoints included overall survival (OS), PFS by blinded independent central review (BICR), overall response rate (ORR) and safety. ESR1-mutation status was centrally determined in blood samples obtained before treatment administration. Patients harboring at least one ESR1 mutation classified as “oncogenic” or “likely oncogenic” were included in the subgroup of patients with ESR1 mutations.
The primary efficacy analysis of PFS showed notable differences based on treatment groups and the presence of genetic mutations, such as ESR1.1,2 Imlunestrant monotherapy demonstrated a 38% reduction in the risk of progression or death for patients with ESR1 mutations compared with ET (HR: 0.62 [95% CI: 0.46–0.82]; p<0.001) (Figure 1). Median PFS was 5.5 months for imlunestrant versus 3.8 months for ET, with consistent benefits observed across subgroups of patients with ESR1 mutations, including those with visceral metastases, prior CDK4/6 inhibitor use or phosphoinositide 3-kinase (PI3K) pathway mutations. However, in the overall population, including patients without ESR1 mutations, imlunestrant monotherapy did not demonstrate a statistically significant PFS improvement compared with ET (HR: 0.87 [95% CI: 0.72–1.04]; p=0.12), with the median PFS being nearly identical between the two groups (5.6 months vs 5.5 months). Subgroup analyses for patients without ESR1 mutations showed no difference in PFS between the treatment arms (HR: 1.00 [95% CI: 0.79–1.27]).
The combination of imlunestrant and abemaciclib proved effective in the overall population, delivering a 43% reduction in the risk of progression or death compared with imlunestrant monotherapy (HR: 0.57 [95% CI: 0.44–0.73]; p<0.001), with a median PFS of 9.4 months versus 5.5 months.1,2 Consistent benefits were observed regardless of ESR1 mutation status and across clinically significant subgroups, including patients with prior CDK4/6 inhibitor treatment and those with PI3K pathway mutations.
OS data remain immature, but early findings suggest a promising trend in favor of imlunestrant versus ET.1,2 Interim OS analysis showed 31% data maturity for the ESR1-mutated group (HR: 0.55 [95% CI: 0.35–0.86]; p=0.008), 23% for the overall population (HR: 0.69 [95% CI: 0.50–0.96]) and 18% for the non-mutated group (HR: 0.87 [95% CI: 0.54–1.40]).
From a safety perspective, imlunestrant monotherapy had a favorable profile, with mostly mild to moderate toxicity.1,2 The most common treatment-emergent adverse events (TEAEs) included fatigue (23% vs 13% with ET), diarrhea (21% vs 12%) and nausea (17% vs 13%), with the majority being grade 1 and single episodes. The rate of grade ≥3 TEAEs was lower with imlunestrant compared with ET (17% vs 21%), as were the rates of dose reductions and discontinuations (2% and 4%, respectively). Importantly, there were no oral SERD-specific toxicities, such as ocular or cardiac safety signals, alleviating concerns regarding potential class-specific safety issues. The combination of imlunestrant with abemaciclib had a safety profile consistent with prior studies and demonstrated low rates of treatment discontinuations (6%), with a manageable safety profile. Grade ≥3 TEAEs were reported in 49% of patients in the combination arm.
In summary, imlunestrant significantly improved PFS compared with standard therapy in patients with advanced ER+/HER2- breast cancer with ESR1 mutations but not in the overall population. Imlunestrant plus abemaciclib significantly improved PFS compared with imlunestrant monotherapy, regardless of the ESR1 mutation status.1,2
PHILA: Pyrotinib, trastuzumab and docetaxel for HER2+ metastatic breast cancer
HER2-positive (HER2+) breast cancer accounts for 15–20% of all breast cancer cases.6–8 Clinical evidence has shown that dual HER2-targeted therapies with complementary mechanisms of action offer a more effective blockade of HER2 signaling than single-agent treatments.8–11 Currently, the preferred first-line regimen per international guidelines is the combination of pertuzumab with trastuzumab and docetaxel, based on the CLEOPATRA study which demonstrated significant survival benefits.12–14 Pyrotinib, a small-molecule reversible pan-ErbB tyrosine kinase inhibitor, represents a novel therapeutic strategy in this setting.15–17
The PHILA study (NCT03863223) was a phase III, randomized, double-blind, multicenter trial conducted at 40 sites in China that evaluated the combination of pyrotinib plus trastuzumab and docetaxel (PyroHT) versus placebo plus trastuzumab and docetaxel (HT) in treatment-naive patients with HER2+ metastatic breast cancer.18 A total of 590 patients were enrolled to receive PyroHT (n=297) or HT (n=293). Interim results from the PHILA trial had demonstrated that the study met its primary endpoint of investigator-assessed PFS, with significantly prolonged PFS in the pyrotinib arm compared with the placebo arm (median, 24.3 months vs 10.4 months; HR: 0.41 [95% CI: 0.32–0.53]; p<0.001). The secondary endpoints included OS, objective response rate (ORR), duration of response (DoR) and safety. At the SABCS 2024, the prespecified final analysis of PFS, as well as long-term efficacy and safety were reported.19
The updated findings from the PHILA study further reinforce the efficacy and safety of PyroHT.19 At a median follow-up of 35.7 months in the PyroHT arm and 34.3 months in the HT arm, the median PFS was 22.1 months versus 10.5 months, respectively (HR: 0.44 [95% CI: 0.36–0.53]; p<0.0001). The 3-year PFS rate was substantially higher at 39.7% in the PyroHT arm versus 9.9% in the control arm. OS was also significantly improved, with a 4-year OS rate of 74.5% compared with 64.3% in the control arm (HR: 0.64 [95% CI: 0.46–0.89]; p=0.0038) (Figure 2). Response rates were robust in the PyroHT group, achieving an ORR of 84.2% and a DoR of 22.3 months compared with 71.7% and 10.4 months in the control arm, respectively.
The safety profile of PyroHT was consistent with previous reports, showing a higher incidence of grade ≥3 treatment-related adverse events (TRAEs) in the PyroHT arm compared with the HT arm (90.9% vs 77.5%), with diarrhea being the most common (grade 3, 47.8% vs 4.4%).19 TRAEs led to treatment discontinuation in 15.2% of the PyroHT arm compared with 7.5% in the control arm. No new safety signals were identified, and the side effects were considered manageable overall.
These final results establish PyroHT as a highly effective first-line treatment option for HER2+ metastatic breast cancer associated with significant improvements in PFS, OS and response rates.19 Despite the higher incidence of AEs, its sustained efficacy and manageable safety profile position PyroHT as a valuable addition to HER2-targeted therapy strategies. Taken together, the CLEOPATRA12,13 and PHILA18 studies demonstarted that dual HER2 inhibition outperforms singular HER2 blockade in patients with HER2+ metastatic breast cancer, confirming the general principle that dual HER2 blockade improves treatment efficacy.
INSEMA: De-escalation of axillary surgery
The INSEMA trial (NCT02466737) was conducted to assess whether axillary surgery could be safely omitted in patients with clinically node-negative early breast cancer who were undergoing breast-conserving surgery.20,21 Its rationale was based on the growing evidence that sentinel lymph node biopsy (SLNB),22,23 which is primarily a diagnostic procedure, may lead to unnecessary surgical complications without significantly impacting long-term outcomes. The de-escalation of axillary surgery was also investigated in the Sentinel Node versus Observation After Axillary Ultra-Sound (SOUND) trial,24 which showed non-inferior disease-free survival (DFS) and OS when SLNB was avoided in favor of axillary imaging for staging. This trial also highlighted the critical role of pre-operative imaging, particularly axillary ultrasound, in reliably identifying patients who could avoid invasive procedures.
This study, conducted across 151 sites in Germany and Austria, included patients with breast cancer with tumor size ≤5 cm (clinical T1–T2), cNo, planned breast-conserving surgery and post-operative irradiation.20,21 Notably, 90.5% of patients had tumor size of ≤2 cm. A total of 4,858 patients were randomized in a 4:1 ratio to either undergo SLNB (n=3,896) or proceed without SLNB (n=962). The primary endpoint was non-inferiority of invasive DFS (iDFS) with no axillary surgery versus the standard SLNB.
The study met its primary endpoint by demonstrating that the omission of SLNB led to non-inferior outcomes compared with that achieved with SLNB.20,21 At five years, the iDFS rate was 91.9% in patients who did not undergo SLNB compared with 91.7% in the SLNB arm (HR: 0.91 [95% CI: 0.73–1.14]) (Figure 3). Additionally, the 5-year OS rate was numerically but not statistically higher in the no-SLNB arm, at 98.2% compared with 96.9% in the SLNB arm.
The INSEMA trial findings suggest that omitting SLNB in carefully selected patients at low-risk does not compromise oncological safety and can substantially reduce surgical morbidity, such as lymphedema and shoulder dysfunction.20,21 Patients aged ≥50 years, with small tumors (≤2 cm), grade 1–2 histology and HR+, HER2- disease appear to be the most suitable candidates for this de-escalated approach.
FASCINATE-N: Neoadjuvant HER2-directed ADC with SHR-A1811 in HER2+ early breast cancer
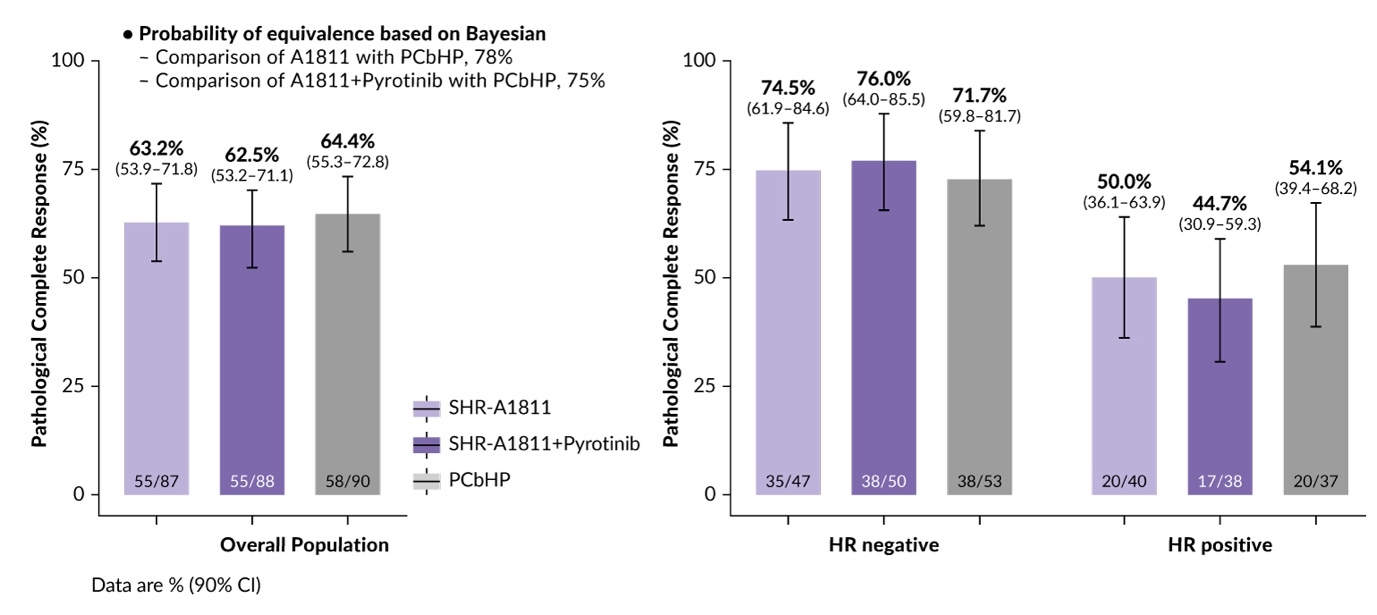
The Fudan University Shanghai Cancer Center Breast Cancer Precision Platform Series study - neoadjuvant therapy (FASCINATE-N) trial (NCT05582499) is a phase II study designed to evaluate the efficacy and safety of SHR-A1811, a third-generation HER2-directed antibody-drug conjugate (ADC),25,26 in patients with early-stage HER2+ breast cancer in the neoadjuvant setting.27 The SHR-A1811 molecule is comprised of a monoclonal antibody trastuzumab, a cleavable linker and topoisomerase 1 inhibitor payload. It has a drug-to-antibody ratio (DAR) of 6 for enhanced anti-tumor activity compared with trastuzumab emtansine (DAR, 3.5) and potentially improved safety profile compared with trastuzumab deruxtecan (DAR, 8).28 In the earlier global phase I trial, administering SHR-A1811 at doses of 4.8 and 6.4 mg/kg every three weeks demonstrated significant efficacy in patients with heavily pretreated HER2+ breast cancer, achieving an overall response rate of 76%. FASCINATE-N enrolled 265 patients with early-stage HER2+ disease and primary tumor size of ≥2 cm.15 Patients were randomized 1:1:1 to receive SHR-A1811 as monotherapy (n=87), SHR-A1811 combined with pyrotinib (n=88) and the nab-paclitaxel, carboplatin, trastuzumab and pertuzumab (PCbHP) regimen consisting of nab-paclitaxel, carboplatin, trastuzumab and pertuzumab (n=90), which is considered a standard neoadjuvant therapy in this patient population. More than 90% of patients in the study population were lymph node-positive and 70% had stage III disease. The primary endpoint was pathological complete response (pCR).
The trial showed promising efficacy results, with similar pCR rates reported for all three treatment regimens, without significant differences between the study arms (Figure 4).27 SHR-A1811 monotherapy achieved a pCR rate of 63.2%, with particularly notable outcomes in the hormone-receptor (HR)-negative group, which had a pCR rate of 74.5%. For the HR+ subgroup, the pCR rate was 50.0%. SHR-A1811 combined with pyrotinib showed a pCR rate of 62.5%, while the PCbHP regimen achieved a pCR rate of 64.4%.
SHR-A1811 demonstrated a favorable safety profile, especially as monotherapy, with fewer TRAEs leading to dose reductions or treatment discontinuations compared with the PCbHP regimen (10.3% vs 13.3% and 5.7% vs 16.7%, respectively).27 However, combining SHR-A1811 with pyrotinib led to increased rates of TRAEs, particularly diarrhea (all grade, 95.5% vs 34.6% with SHR-A1811 monotherapy vs 51.1% with PCbHP).
In summary, FASCINATE-N is the first study to report the efficacy and safety of a third-generation HER2-targeting ADC as a neoadjuvant therapy for HER2+ breast cancer.27 Comparable efficacy and encouraging safety profile of SHR-A1811 monotherapy position this ADC as a backbone and combination with targeted therapies marking the era of the so-called “ADC+”.
CamRelief: Neoadjuvant camrelizumab plus chemotherapy in TNBC
Triple-negative breast cancer (TNBC), representings around 15% of all breast cancer cases, is known for its aggressive nature, high recurrence rates and poor survival outcomes.29 Being the most immunogenic breast cancer subtype, TNBC is particularly suited for immunotherapy approaches.30 The current standard of care for neoadjuvant treatment in patients with TNBC is pembrolizumab plus platinum-containing chemotherapy followed by adjuvant pembrolizumab, based on the findings from the phase III KEYNOTE-522 trial.31,32 This study demonstrated significantly improved pCR rates and OS with the addition of pembrolizumab to platinum-containing chemotherapy.
Camrelizumab, an anti-programmed cell death protein 1 (PD-1) monoclonal antibody, has shown strong antitumor activity and tolerability in prior phase II studies in TNBC.33,34 The phase III CamRelief trial (NCT04613674) was designed to evaluate the efficacy and safety of neoadjuvant camrelizumab in combination with chemotherapy compared with chemotherapy alone for early or locally advanced TNBC.35,36 This multicenter trial included 441 patients with stage II–III invasive disease and no prior systemic therapy who were randomized to receive chemotherapy in combination with either camrelizumab (n=222) or placebo (n=219).34 The chemotherapy regimen included nab-paclitaxel and carboplatin for the first 16 weeks, followed by a dose-dense anthracycline-cyclophosphamide (ddAC) regimen every two weeks for eight weeks, as this combination had previously demonstrated the ability to improve pCR rates, a surrogate marker for long-term survival. The primary endpoint was the pCR rate, defined as the absence of invasive tumors in the breast and lymph nodes at surgery. The secondary endpoints included event-free survival (EFS), DFS, distant disease-free survival (DDFS), pre-surgery ORR and safety.
Camrelizumab combined with chemotherapy significantly improved the pCR rate compared with chemotherapy alone (Figure 5).35,36 At a median follow-up of 14.4 months, the pCR rate was 56.8% in the camrelizumab group versus 44.7% in the placebo group. The subgroup analysis showed promising outcomes for high-risk stage III TNBC (pCR rate of 49.4% vs 38.0% with chemotherapy) and node-positive TNBC (57.8% vs 42.7% with chemotherapy). Moreover, early trends in EFS, DFS and DDFS support the benefits of camrelizumab plus chemotherapy (HR: 0.58–0.80).
The safety profile of the combination was manageable and consistent with the known profiles of each agent.35,36 Grade ≥3 AEs occurred in 89.2% of patients in the camrelizumab arm compared with 83.1% in the placebo arm, with serious AEs reported in 34.7% and 22.8% of patients, respectively. Fatal AEs were observed in two patients in the camrelizumab group versus none in the placebo group. The most common AEs included decreased neutrophil, platelet and white blood cell counts.
In conclusion, camrelizumab plus chemotherapy represents a potential new option in early or locally advanced TNBC, especially given its immunogenic profile and limited targeted therapies for this aggressive disease subtype.35,36 Notably, compared with the KEYNOTE-522 study, the CamRelief study included a higher proportion of high-risk patients, including those with N3 disease, which led to a slightly lower pCR rate (56.8% vs 64.8%).31,35 The trials also differed in neoadjuvant chemotherapy regimens, as CamRelief used nab-paclitaxel instead of conventional paclitaxel, with weekly nab-paclitaxel plus carboplatin administered over 16 weeks versus 12 weeks in KEYNOTE-522. In addition, the CamRelief trial employed a ddAC regimen, whereas KEYNOTE-522 used a three-weekly schedule.
EBCTCG Analysis: Obesity and prognosis in early-stage breast cancer
Obesity is known to be associated with worse outcomes in breast cancer, but the strength of this link varies according to menopausal and ER status. The primary aim of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) analysis was to investigate the relationship between obesity, measured by body mass index (BMI) and breast cancer prognosis, with a focus on the risk of distant recurrence and mortality in early stage breast cancer.
This large-scale study analyzed data from 206,904 women enrolled in 147 randomized clinical trials.37 Among the patients, 26% were classified as obese (BMI: ≥30), 60% were postmenopausal and 77% had ER+ tumors. BMI measurements collected within two years of diagnosis ranged from 15 to 50 kg/m². The patients were categorized into five groups: underweight (BMI: <20), normal weight (BMI: 20–25), overweight (BMI: 25–30), obese (BMI: 30–35) and obesity class 1 (BMI: ≥35). Cox regression analyses were used to evaluate the association between BMI and breast cancer outcomes, adjusting for factors such as treatment type, age, tumor characteristics and trial variables.
The analysis revealed a linear relationship between BMI and the risk of distant recurrence and mortality.37 For every 5-unit increase in BMI, the adjusted risk of distant recurrence increased by 6% (rate ratio [RR]: 1.06) (Figure 6). The risk difference was particularly notable when comparing obese (BMI ≥30) and underweight (BMI <20) women, with a 17% higher risk of distant recurrence (RR: 1.17) in obese women.
The strength of the association between BMI and distant recurrence was independent of systemic therapy and ER status, with BMI being equally relevant in ER+ and ER- disease.37 However, the effect of obesity was slightly higher in premenopausal women compared with postmenopausal patients. The absolute 10-year excess risk of distant recurrence was 2% for node-negative (N0) disease and 4% for node-positive (N+) disease in premenopausal women, compared with 2% and 1%, respectively, in postmenopausal women. Similar trends were observed for breast cancer mortality.
Together, the continuous association of BMI with distant recurrence rate underscores the importance of weight management in breast cancer care.37 While the absolute reduction in risk with weight loss is modest (~1% for a 5 kg/m² BMI drop), these findings emphasize the value of addressing obesity in clinical practice to improve both breast cancer outcomes and overall health.
AI-driven risk prediction for primary prevention
Although image-based artificial intelligence (AI) risk models have shown promise in short-term risk assessment for improving breast cancer screening, no image-derived long-term AI risk model for primary prevention existed to date. The main goals of the study conducted through the Mayo-Karolinska collaboration were to develop and externally validate an image-derived AI-based risk model to predict the risk of breast cancer across 10 years of follow-up and compare the new tool with the traditional Tyrer-Cuzick v8 risk model.38 The study utilized data from over 10,143 women in case-cohorts collected in Minnesota, USA, between 2009 and 2017 and Sweden between 2010 and 2013. The model, built with an ensemble machine learning approach, combined mammographic image features, age and incidence rates to predict 10-year breast cancer risk.
The image-derived AI-based risk model demonstrated superior performance compared with the Tyrer-Cuzick model.38 It showed better discriminatory accuracy, improved calibration and more effective classification of clinical risk. The AI model performed consistently across various subgroups, including different age ranges (<50, 50–69 and ≥70 years), racial categories (white and non-white women) and breast cancer subtypes (ER+ and ER-). Approximately 40% of breast cancer cases were identified at study entry among the 15–18% of women deemed high-risk per United States Preventive Services Task Force (USPSTF) clinical guidelines.
By accurately identifying women at high risk, these models can guide early interventions, including lifestyle changes and ETs, to reduce breast cancer incidence.38 The ability to generalize across diverse populations and screening settings further enhances the clinical value of this AI-based approach, paving the way for its integration into routine screening programs and informing preventive healthcare decisions.
PADMA: Palbociclib and endocrine therapy in HR+/HER2- metastatic breast cancer
The PADMA trial (NCT03355157) was a prospective, randomized, open-label, multicenter phase III study designed to compare the efficacy of palbociclib plus ET versus standard mono-chemotherapy (with or without ET maintenance) as first-line treatment for high-risk HR+, HER2- metastatic breast cancer.39 The trial addressed a gap in predictive data comparing ET with the SoC chemotherapy, despite international guidelines already advocating for the use of CDK4/6 inhibitors alongside ET. The trial enrolled patients with HR+, HER2- metastatic disease who were untreated for metastatic or relapsed disease and required chemotherapy. Exclusion criteria were asymptomatic bone-only disease, untreated central nervous system (CNS) metastases and life expectancy under six months. A total of 150 patients were randomized to receive either palbociclib with ET or physician’s choice of chemotherapy (e.g., capecitabine, paclitaxel), and the primary endpoint was time to treatment failure (TTF).
The PADMA trial met its primary endpoint, demonstrating significantly improved TTF outcomes for palbociclib plus ET over chemotherapy.39 At a median follow-up of 36.8 months, the median TTF was 17.2 months for the palbociclib plus ET arm versus 6.1 months for the chemotherapy arm (HR: 0.46 [95% CI: 0.31–0.69]; p<0.001) (Figure 7). The median PFS was 18.7 months with palbociclib plus ET versus 7.8 months with chemotherapy (HR: 0.45 [95% CI: 0.29–0.70]; p<0.001), with a trend toward improved OS in the palbociclib plus ET arm (46.1 months vs 36.8 months).
While the rates of hematologic toxicity were higher with palbociclib plus ET, the safety profile remained manageable, and no new safety signals were detected.39 These findings support current guidelines recommending the use of ET with a CDK4/6 inhibitor as the standard first-line treatment for HR+, HER2- metastatic breast cancer, offering a more effective alternative to chemotherapy.
Conclusion: Toward Precision and Personalized Care
The SABCS 2024 served as an important platform for advancing breast cancer management and precision medicine. From the potential of imlunestrant in endocrine resistance to AI-driven imaging tools and effective therapeutic innovations across HER2+ and TNBC subtypes, the symposium cemented its place as a frontrunner in advancing breast cancer care. The integration of these advances into clinical practice will redefine treatment paradigms, offering hope for more effective, less burdensome, and highly personalized patient journeys in the fight against breast cancer.
Conflict of interest
Marcus Vetter received consulting fees from GSK, Roche, Novartis, ExactSciences, Pfizer, Stemline, AbbVie and ASC Oncology. These funding entities did not play any role in the development of the manuscript and did not influence its content in any way. Other authors have declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors have declared that no financial support was received from any organization for the submitted article.
Author contributions
All authors contributed to and approved the final manuscript.
_endocrine_therapy.jpeg)
_results_for_pyrotinib_plus_trastuzumab_and_docetaxel_(pyroht.jpeg)
_in_patients_with_early_breast_cancer_with_.jpeg)


_in_the_early_breast_cancer_trialists__collaborative_gro.jpeg)
_in_the_padma_trial.jpeg)
_endocrine_therapy.jpeg)
_results_for_pyrotinib_plus_trastuzumab_and_docetaxel_(pyroht.jpeg)
_in_patients_with_early_breast_cancer_with_.jpeg)

_in_the_early_breast_cancer_trialists__collaborative_gro.jpeg)
_in_the_padma_trial.jpeg)