Introduction
Prostate cancer is the second most prevalent malignancy and the fifth leading cause of death among men globally.1 The disease progresses through multiple stages, starting with localized, organ-confined cancer and advancing to metastatic phases, which are classified by hormonal responsiveness. In localized, early-stage prostate cancer, the prognosis is generally favorable, with a 10-year overall survival (OS) rate of 98%.1,2 Treatment options for localized prostate cancer include active surveillance, ablative radiotherapy and radical prostatectomy.3 However, the prognosis worsens dramatically when the disease progresses to metastatic stages, characterized by tumor cell dissemination mostly to the bone and lymph nodes, resulting in a 5-year OS rate of only 30%.1,4
As androgen receptor signaling is crucial for tumor growth and survival in the metastatic stage, androgen deprivation therapy (ADT) has been the standard of care (SoC) in patients with metastatic hormone-sensitive prostate cancer (mHSPC).3,5 Combining ADT with androgen receptor pathway inhibitors (ARPIs) has further improved patient outcomes. In mHSPC, chemotherapy and the combination of ARPI with chemotherapy also significantly prolong OS. Although most patients initially respond to ADT, many will eventually develop castration resistance, which leads to androgen-independent tumor growth and signifies a transition to the more aggressive, incurable state of metastatic castration-resistant prostate cancer (mCRPC), associated with a poor prognosis and a mean survival of only 16–18 months.6,7 In recent years, several classes of novel drugs have been introduced into clinical practice, including ARPIs, taxanes, poly (ADP-ribose) polymerase (PARP) inhibitors and immunotherapies, which have demonstrated significant survival benefits for these patients.3,8–11
Recent advancements in prostate cancer treatment include the development of therapies targeting prostate-specific membrane antigen (PSMA). PSMA is a type II transmembrane glycoprotein encoded by the folate hydrolase 1 (FOLH1) gene,12 which is present on the benign prostate epithelium and other tissues. Its expression is markedly upregulated in prostate adenocarcinoma cells, including metastatic lesions in mCRPC patients, correlating with poor prognosis.13–16
As a transmembrane protein with a known substrate, PSMA is overexpressed on prostate cancer cells and internalizes upon ligand binding, making it an attractive target for small-molecule ligands designed for both diagnostic and therapeutic purposes. This combined approach, known as theranostics, utilizes radiotracers that selectively bind to PSMA, such as 68Ga-PSMA-11, for positron emission tomography (PET) imaging of PSMA-positive disease.17 Therapeutically, radioligands, such as 177Lutetium (Lu)-PSMA-617 (177Lu-PSMA-617), have shown potential as a radionuclide therapy option for targeted delivery of β-radiation to PSMA-expressing tumor cells, causing DNA damage and cytotoxicity.18,19 This dual approach thus enables visualization and selective treatment of prostate cancer at advanced stages.
PSMA radioligand therapy: The state of the art
The VISION trial: 177Lu-PSMA-617 improves rPFS and OS in patients with mCRPC
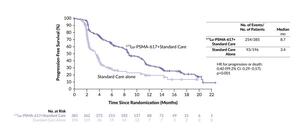
The registrational, open-label VISION trial investigated 177Lu-PSMA-617 in 831 patients with mCRPC who had previously received at least one ARPI and one to two taxane regimens and had PSMA-positive 68Ga-PSMA-11 PET/computed tomography (CT) scans.20 At a median follow-up of 20.9 months, patients treated with 177Lu-PSMA-617 plus SoC versus SoC alone achieved significantly improved radiographic progression-free survival (rPFS) (median: 8.7 months vs 3.4 months; HR: 0.40 [99.2% CI: 0.29–0.57]; p<0.001) (Figure 1) and OS (median: 15.3 months vs 11.3 months; HR: 0.62 [95% CI: 0.52–0.74]; p<0.001).
In terms of safety, treatment with 177Lu-PSMA-617 was generally manageable.20 Hematologic grade ≥3 adverse events (AEs) were more frequent with 177Lu-PSMA-617 plus SoC than SoC alone and most commonly included anemia (12.9% vs 4.9%), thrombocytopenia (7.9% vs 1.0%) and lymphopenia (7.8% vs 0.5%). Further analyses of the VISION study showed that 177Lu-PSMA-617 plus SoC delayed the time to worsening of health-related quality of life (HRQoL) compared with SoC alone.21 In particular, 177Lu-PSMA-617-containing treatment was associated with prolonged time to worsening of the FACT-P score (HR: 0.54), BPI-SF pain intensity score (HR: 0.52) and EQ-5D-5L utility score (HR: 0.65). 177Lu-PSMA-617 also prolonged the time to first skeletal event or death (median: 11.5 months vs 6.8 months with SoC alone; HR: 0.50).
The recent VISION dosimetry sub-study, which quantified absorbed doses of 177Lu-PSMA-617 in the kidneys and other organs, showed that 177Lu-PSMA-617 had a good safety profile with low radiotoxicity in organs at risk.22 A further analysis of tumor dosimetry for 177Lu-PSMA-617 across cycles 1–6 was conducted in a cohort of 29 non-randomized patients who received up to six cycles of 177Lu-PSMA-617 (7.4 GBq every six weeks) plus SoC.23 Patients underwent single-photon emission computed tomography (SPECT)/CT scans to assess tumor uptake and calculate radiation-absorbed doses over time. Data from 60 delineated prostate cancer lesions were analyzed across cycles 1–6 in 18 patients who had evaluable tumors in cycles 2–6. The mean absorbed dose across all tumors decreased from 7.9 Gy/GBq in cycle 1 to 1.6 Gy/GBq by cycle 6, indicating reduced radiation delivery to tumors over successive cycles. Mean absorbed doses decreased from 6.4 Gy/GBq in cycle 1 to 1.3 Gy/GBq in cycle 6 for bone tumors and from 11 Gy/GBq in cycle 1 to 3.2 Gy/GBq in cycle 6 for lymphatic metastases. Patients received a median cumulative absorbed dose of approximately 100 Gy over six cycles. The declining absorbed doses across cycles 1–6 in this dosimetry sub-study align with the demonstrated anti-tumor efficacy of 177Lu-PSMA-617 observed in the VISION trial.
TheraP: 177Lu-PSMA-617 yields higher PSA50 rates versus cabazitaxel
Another trial assessing the efficacy of 177Lu-PSMA-617 against cabazitaxel in patients with mCRPC for whom cabazitaxel was considered the next appropriate standard treatment was the randomized phase II TheraP trial, which included 200 patients.24 The study met its primary endpoint of prostate-specific antigen (PSA) response, defined as a reduction of at least 50% from baseline (PSA50), with significantly higher PSA50 rates under 177Lu-PSMA-617 compared with cabazitaxel (66% vs 37% by intention-to-treat; p<0.0001). A pre-specified sensitivity analysis by treatment received showed a PSA50 response in 66% of men treated with 177Lu-PSMA-617 versus 44% of those treated with cabazitaxel (p=0.0016).
Treatment with 177Lu-PSMA-617 also delayed disease progression compared with cabazitaxel in terms of both rPFS (HR: 0.64 [95% CI: 0.46–0.88]; p=0.0070) and PSA PFS (HR: 0.60 [HR: 0.44–0.83]; p=0.0017). At a median follow-up of 36 months, OS analysis by intention-to-treat, summarized as restricted mean survival time (RMST), demonstrated similar OS between patients receiving 177Lu-PSMA-617 and cabazitaxel (RMST, 19.1 months vs 19.6 months; p=0.77).25 In a safety analysis (n=183), grade 3–4 AEs occurred in 33% of men in the 177Lu-PSMA-617 group and 53% in the cabazitaxel group.
Based on the results from the VISION and TheraP studies, both the European Association of Urology (EAU) and the American Society of Clinical Oncology (ASCO) recommend 177Lu-PSMA-617 for patients with pretreated mCRPC who show high PSMA expression (exceeding the uptake in the liver) on the diagnostic radiolabeled PSMA PET/CT scan.26,27
PSMAfore: Moving to earlier lines of therapy
More recent research has demonstrated that 177Lu-PSMA-617 is also an effective treatment option for mCRPC in earlier lines of therapy. The randomized, open-label, phase III PSMAfore study evaluated the efficacy of 177Lu-PSMA-617 in taxane-naïve patients with mCRPC who had progressed once after a prior ARPI.28 In this study, 468 patients underwent 1:1 randomization to receive either 177Lu-PSMA-617 at a dosage of 7.4 GBq once every six weeks for six cycles or a change of ARPI (abiraterone or enzalutamide).
In the primary analysis, with a median follow-up of 7.26 months, the median rPFS was 9.30 months in the 177Lu-PSMA-617 group compared with 5.55 months in the ARPI change group (HR: 0.41 [95% CI: 0.29–0.56]; p<0.0001).28 An updated analysis at a median follow-up of 24.11 months confirmed this benefit, showing a median rPFS of 11.60 months for the 177Lu-PSMA-617 group versus 5.59 months for the ARPI change group (HR: 0.49 [95% CI: 0.39–0.61]) (Figure 2). Regarding safety, the incidence of grade 3–5 AEs was lower with 177Lu-PSMA-617, with at least one event occurring in 36% of patients, including four grade 5 events (none of them treatment-related). In comparison, 48% of patients receiving ARPI change had grade 3–5 AEs, including five (2%) grade 5 events (one of them treatment-related).
UpFrontPSMA: 177Lu-PSMA-617 as a treatment option for mHSPC
Ample evidence shows that 177Lu-PSMA-617 improves survival and QoL in patients with mCRPC, but its benefit in hormone-sensitive disease has only been investigated recently. In this context, the phase II UpFrontPSMA study aimed to evaluate the impact of 177Lu-PSMA-617 prior to the introduction of docetaxel treatment in adult patients with de novo high-volume metastatic hormone-sensitive prostate cancer (mHSPC).29 In total, 130 patients were randomly assigned in a 1:1 manner to receive either ¹⁷⁷Lu-PSMA-617 (two cycles of 177Lu-PSMA-617 at 7.5 GBq every six weeks) followed by docetaxel six weeks later (every three weeks) versus SoC and docetaxel alone (six cycles every three weeks). All patients received continuous ADT.
The primary endpoint of undetectable PSA (≤0.2 ng/mL) at 48 weeks was achieved in 41% of patients in the 177Lu-PSMA-617 plus docetaxel group compared with 16% of patients in the docetaxel alone group (OR: 3.88 [95% CI: 1.61–9.38]; p=0.0020).29 Additionally, undetectable PSA at any time was observed in 51% of patients receiving the combination therapy versus 32% of those receiving docetaxel alone (OR: 2.14 [95% CI: 1.03–4.46]; p=0.042). The median PSA PFS was 31 months in the combination group versus 20 months in the docetaxel alone group (HR: 0.60 [95% CI: 0.37–0.98]; p=0.039) and the median rPFS was not reached versus 22 months, respectively (HR: 0.58 [95% CI: 0.32–1.05]; p=0.067). The median OS was not reached in either arm (HR: 0.83 [95% CI: 0.38–1.83]; p=0.646). In terms of safety, the most common grade 3 or 4 treatment-related AEs were febrile neutropenia (11% with combination therapy vs 10% with docetaxel alone) and diarrhea (6% vs 0%). Serious adverse events occurred in 25% of patients in each treatment arm.
ENZA-p: Adding enzalutamide to 177Lu-PSMA-617 improves clinical outcomes
Enzalutamide and 177Lu-PSMA-617 both improve OS in patients with mCRPC,20,30,31 and targeting both androgen and PSMA receptors simultaneously might provide complementary benefits due to their close intracellular relationship. The combination of enzalutamide and 177Lu-PSMA-617 was therefore assessed as the first-line treatment for high-risk mCRPC in the phase II ENZA-p trial.32 In this study, 162 patients were randomized to receive either enzalutamide (160 mg daily) plus dose-adapted 177Lu-PSMA-617 (two or four doses; 7.5 GBq 177Lu-PSMA-617 every 6–8 weeks) or enzalutamide alone.
At a median follow-up of 20 months, the median PSA PFS was 13.0 months in the combination arm (n=83) and 7.8 months in the enzalutamide-alone arm (n=79) (HR: 0.43 [95% CI: 0.29–0.63]; p<0.0001).32 The PSA90 reduction rate was also considerably higher with enzalutamide plus 177Lu-PSMA-617, with 78% of patients achieving a 90% reduction in PSA levels compared with 37% with enzalutamide alone. The recently reported secondary endpoint of OS favored the combination treatment, with a median of 34 months versus 26 months for enzalutamide alone (HR: 0.55 [95% CI: 0.36–0.84]; p=0.0053).33
Among 162 patients with available HRQoL data, enzalutamide plus 177Lu-PSMA-617 significantly improved deterioration-free survival at 12 months compared with enzalutamide alone for both physical function (median, 10.64 months vs 3.42 months; HR: 0.51 [95% CI: 0.36–0.72]; p<0.0001) and overall health and QoL (median, 8.71 months vs 3.32 months; HR: 0.47 [95% CI: 0.33–0.67]; p=0.0001).33 Additionally, pain and fatigue scores favored the combination treatment (pain difference: 7.3; p=0.012, fatigue difference: 5.9; p=0.016).
Safety analysis showed that grade 3–5 AEs occurred in 40% of patients in the enzalutamide plus 177Lu-PSMA-617 group and 41% of patients in the enzalutamide group.32 Grade 3 AEs that occurred only in the combination group included anemia (4%) and decreased platelet count (1%).
SPLASH: 177Lu-PNT2002 prolongs rPFS in mCRPC patients
177Lu-PNT2002 (177Lu-PSMA-I&T) is another Lu-PSMA-containing radio-conjugate that has demonstrated significant clinical activity in patients with mCRPC following ARPI therapy. In the phase III SPLASH trial, 412 patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PNT2002 at 6.8 GBq once every eight weeks for up to four cycles or an alternate ARPI (enzalutamide or abiraterone acetate).34
The study met its primary endpoint, showing a median rPFS of 9.5 months in the 177Lu-PNT2002 arm compared with 6.0 months in the ARPI arm, at a median follow-up of 11.1 months and 12.9 months, respectively (Figure 3).34 This corresponded to a significant 29%-reduction in the risk of progression or death (HR: 0.71 [95% CI: 0.55–0.92]; p=0.0088). Treatment with 177Lu-PNT2002 also led to a significantly improved best overall response rate (ORR) at 38.1% in the 177Lu-PNT2002 arm and 12% in the ARPI arm (p=0.0021), with a median duration of response of 9.4 months versus 7.3 months, respectively. The complete response (CR) rate was 9.3% with 177Lu-PNT2002, while no CRs were observed with ARPI. In the 177Lu-PNT2002 arm, 35.7% of evaluable patients achieved at least a 50% PSA decrease from baseline, compared with 14.6% in the ARPI arm.
177Lu-PNT2002 also demonstrated a favorable safety profile.34 Grade ≥3 treatment-emergent AEs occurred in 30.1% of patients in the 177Lu-PNT2002 arm and 36.9% in the ARPI arm, with treatment-related grade ≥3 treatment-emergent AEs (TEAEs) of 9.7% and 11.5%, respectively. Serious TEAEs were reported in 23.1% of patients in the 177Lu-PNT2002 arm versus 17.1% in the ARPI arm, with treatment-related serious TEAEs occurring in 2.2% of 177Lu-PNT2002 patients compared with 3.8% of ARPI patients. The full publication of the SPLASH trial is still awaited.
While the SPLASH trial shares many similarities with the PSMAfore trial, as both compare PSMA-targeted therapy with ARPI treatment, key differences exist. Both studies confirm the superiority of PSMA-targeted radioligand therapy over ARPI treatment in mCRPC patients, but differences in dosing, treatment cycles and clinical outcomes provide important insights.28,34 SPLASH evaluated 177Lu-PNT2002 at 6.8 GBq every eight weeks for four cycles, demonstrating a median rPFS of 9.5 months versus 6.0 months with ARPI (HR: 0.71).34 In contrast, PSMAfore investigated 177Lu-PSMA-617 at 7.4 GBq every six weeks for six cycles, which led to a slightly longer median rPFS of 11.6 months in the updated analysis compared with 5.59 months with ARPI (HR: 0.49).28 Safety profiles also varied slightly, with grade ≥3 TEAEs occurring in 30.1% of SPLASH patients versus 36% in PSMAfore, although ARPI arms in both trials had higher toxicity rates.28
SatisfACtion: an ongoing study of 225Ac-PSMA-R2
Newly developed compounds emitting α particles, such as Actinium-225 (225Ac), are showing promise due to their potential to cause more significant DNA damage in tumor cells by a significantly increased number of double-strand breaks compared to β-emitters.35 Actinium-225 conjugated to radio-labeled PSMA-R2 (225Ac-PSMA-R2) is currently under investigation in the SatisfACtion study in patients with PSMA-positive mCRPC who have previously undergone ARPI and taxane therapy.36 In this phase I/II study, patients receive intravenous 225Ac-PSMA-R2 plus SoC once every six weeks for up to six cycles. The dose-escalation phase evaluates the safety, tolerability and recommended dose for expansion of 225Ac-PSMA-R2. Dose escalation is performed in each group using independent Bayesian logistic regression models following the escalation with the overdose control (EWOC) principle. The initial dose of 7 MBq may be increased to 10, 12 or 14 MBq if the EWOC criteria are met and safety profiles remain acceptable. Primary endpoints in the dose-escalation phase include the incidence and severity of dose-limiting toxicities, AEs and serious AEs, dose intensity and treatment modifications. In the dose-expansion phase, the primary endpoints are ORR and PSA50 response rate. Secondary endpoints in both phases include response rates, rPFS, PFS, OS, the time to symptomatic skeletal event, among others.
Patient selection for radioligand therapy
177Lu-PSMA-617 is an effective treatment providing longer survival with improved QoL in patients with mCRPC who have previously received ARPI and taxane therapy. However, as shown above, patient response to 177Lu-PSMA-617 treatment varies and it is therefore crucial to identify those who most likely benefit from such a treatment. The VISION and TheraP trials used different criteria for patient selection based on PSMA-PET.37,38
In the VISION trial, patients had to have PSMA-positive disease based on 68Ga-PSMA-11 staging PET/CT scans, defined as a greater uptake in metastatic lesions than in the liver, and the absence of PSMA-negative metastatic lesions.39 Using these criteria, 13% of the screened men were deemed trial-ineligible, while 46% of the remaining and treated patients achieved a PSA50 response with radioligand therapy. Conversely, the TheraP trial required high PSMA expression with at least one site with a standardized uptake value (SUV)max >20, which was approximately 4–5 times higher than that required for patients in the VISION trial, and no sites of 18F-fluorodeoxyglucose (FDG)-positive/PSMA-negative disease.24 Due to these more stringent criteria, 31% of screened patients were ineligible, while 65% of the treated patients achieved a PSA50 response. These data highlight that the correct application of imaging parameters can significantly influence the treatment choice and response rates to radionuclide therapy.
As PSMA-PET has emerged as a reference imaging tool for the staging and restaging of patients with prostate cancer for both clinical routine and trials, the recently developed PROMISE V2 criteria have been proposed as a set of guidelines designed to standardize the assessment of prostate cancer using updated molecular imaging TNM staging, improved assessment of local disease and a slightly modified PSMA-expression score for clinical routine.40 Key points include standardized imaging protocols, lesion characterization based on specific imaging features and a structured reporting system to facilitate communication among healthcare professionals.
Ad hoc analyses of both VISION and TheraP demonstrated that a high SUV at baseline is associated with superior event-free survival (EFS) and OS.37,38 In VISION, patients in the top quartile (SUVmean ≥9.9) experienced the greatest benefit from treatment, while those with SUVmean <9.9 showed no substantial difference in response rates. In TheraP, patients with SUVmean ≥10 achieved higher PSA50 response rates and PSA PFS compared with those with SUVmean <10 (response rates, 91% vs 52%; HR: 0.45 vs 0.77) (Figure 4). Notably, SUVmean ≥10 was predictive of favorable general response in patients, regardless of the receipt of cabazitaxel or PSMA radioligand therapy in the TheraP trial.
Both the VISION and TheraP trials reported that 22% of patients had SUVmean >10.37,38 However, the “gap” between these two trials, accounting for approximately 17% of patients who failed screening in TheraP but passed in VISION, raises questions about selection criteria. Analysis of this cohort showed that 39% of patients achieved a PSA50 response,41 suggesting that patients with an SUVmean ≤10 should not necessarily be excluded from radioligand therapy.
The role of FDG-PET in patient selection was investigated in an ad hoc analysis of TheraP, which showed its predictive value, with screen failure indicating significantly worse responses.37 However, FDG-PET is not recommended for routine use across all patients, as it may only identify a small number beyond what PSMA-PET alone would detect. Patients should be selected for PSMA radioligand therapy based on an uptake greater than that in the liver. While showing prognostic value, SUVmean >10 is not relevant for clinical decision-making in the current patient population, although it may be more relevant earlier in the disease course. Furthermore, nomograms have proven unreliable for identifying patients who respond to PSMA radioligand therapy and are therefore not recommended for clinical use. Post-chemotherapy, patients should be given the opportunity to undergo radioligand therapy whenever feasible.
Further multivariable models have been developed of OS, rPFS and PSA50 response after treatment with 177Lu-PSMA-617, using data from the VISION trial.42 In single pretreatment parameter analyses, 22 parameters (76%) were associated with OS, 21 (72%) with rPFS and 10 (34%) with PSA50 in the overall VISION population. More specifically, the OS nomogram included SUVmax, time since diagnosis, opioid analgesic use, aspartate aminotransferase (AST), hemoglobin, lymphocyte count, presence of PSMA-positive lesions in lymph nodes, lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and neutrophil count. The rPFS nomogram included SUVmax, time since diagnosis, opioid analgesic use, lymphocyte count, presence of liver metastases by computed tomography, LDH and ALP, while for PSA50, included factors were SUVmax, lymphocyte count and ALP. Higher levels of ALP, AST and LDH, along with the presence of liver metastases, were associated with poorer OS, rPFS and PSA50 outcomes. Conversely, higher hemoglobin levels, neutrophil counts, SUVmean and SUVmax, and longer time since prostate cancer diagnosis correlated with improved OS, rPFS and PSA50. Notably, higher SUVmax or SUVmean was associated with higher PSA50 response rates in the 177Lu-PSMA-617 plus SoC group, although no specific parameters significantly predicted improved OS or rPFS in this treatment arm versus the SoC arm. In multivariable modeling, Spearman’s rank correlation analysis identified co-linearity in three pairs of parameters: neutrophil-to-lymphocyte ratio (NLR) with pan-immune-inflammation value; neutrophil count with white blood cell count; and SUVmean with SUVmax. These models show that a combination of pretreatment laboratory, clinical and imaging parameters, reflecting both patient and tumor status, influences the outcomes.
Repeated imaging with post-treatment SPECT can effectively evaluate PSMA uptake in patients undergoing 177Lu-PSMA-617 therapy.43 Patient response evaluated by PSA levels, imaging data and other factors, such as patient symptoms and toxicities, can be used to guide decisions on delaying or discontinuing treatment. Since the radionuclide 177Lu emits gamma photons, SPECT imaging can track patients over time without the need for further PSMA-PET, helping to monitor response and adjust therapy. As shown in the RE-SPECT study, patients who showed a response may benefit from treatment delays, sparing additional therapy in early responders, decreasing toxicity and potentially extending OS (Figure 5).44 For those with heterogeneous responses, post-treatment scans can be used to determine disease progression before stopping treatment. In fact, one study demonstrated that post-treatment SPECT led to changes in disease management in 49% of patients, suggesting that it may be a more effective approach than PSMA-PET for managing immediate post-treatment responses.45 However, SPECT imaging in clinical routine remains qualitative, while PET imaging provides quantitative imaging data for patient- and lesion-level response assessment, as well as quantitative prognostic data. Conventional imaging techniques, such as CT and magnetic resonance imaging (MRI), are still required to detect the development of PSMA-negative disease.
Use of radioligand therapy in special conditions
In the VISION trial, which included patients with compromised bone marrow function, the exposure-adjusted incidence of TEAEs per 100 patients-treatment years was generally similar in patients receiving 177Lu-PSMA-617 and those receiving SoC.46 Hematologic grade ≥3 TEAEs included anemia (16.7% with 177Lu-PSMA-617 vs 14.0% with SoC) and thrombocytopenia (10.0% vs 2.8%); neutropenia and acute kidney injury were uncommon (4.3% vs 1.4% and 3.8% vs 6.9%). Additionally, a multicenter retrospective study evaluating the efficacy and safety of 177Lu-PSMA-617 in 390 patients, 43 of whom had diffuse bone marrow involvement, reported a PSA50 response rate of 58%, with rates of grade 3 hematologic AEs similar to those observed in VISION (anemia, 22%; thrombocytopenia, 17%).47 These data suggest that patients with bone marrow involvement can generally be safely treated with radioligand therapy.
In managing bone marrow toxicity associated with 177Lu-PSMA-617 therapy, it is important to distinguish between pre-existing and treatment-related bone marrow compromise. In patients with high-volume disease, higher doses of radioactivity may be required to ensure a suitable environment for future bone marrow cell growth. This is because standard doses (7.4 GBq of 177Lu-PSMA-617) may not achieve sufficient therapeutic concentrations in high-volume disease due to a dilution effect. A phase I/II study demonstrated that dose fractionation up to a maximum of 22.0 GBq led to higher cumulative radiation doses, a decreased frequency and depth of PSA response, as well as increased OS and toxicity with higher treatment activities.48
Tumor heterogeneity also impacts response to radioligand therapy and should therefore be considered during patient selection. A novel visually calculated score incorporating heterogeneity and intensity of tumors classified mCRPC patients into four categories based on SUVmax and tumor intensity: (1) SUVmax <15, (2) SUVmax 15–79 with heterogeneous intensity, (3) SUVmax 15–79 with homogenous intensity and (4) SUVmax ≥80.49 Data from different studies demonstrated that a higher heterogeneity index is associated with poor response to 177Lu-PSMA-617 (Figure 6). Patients with highly heterogeneous tumors may experience grade 1–2 anemia and disease progression after treatment, which increase their risk of bone marrow impairment.
Renal toxicity data for 177Lu-PSMA-617 are limited, particularly from prospective studies. A retrospective analysis of 106 patients receiving a minimum of four doses of 177Lu-PSMA-617 reported that the estimated glomerular filtration rate (eGFR) decreased progressively, reaching a 19.6% reduction at 24 months.51 The risk factors associated with impaired eGFR included hypertension, diabetes, older age and prior taxane chemotherapy. In patients with pre-treatment chronic kidney failure, 177Lu-PSMA-617 therapy did not lead to a detectable deterioration in renal function, suggesting that such patients should not be categorically excluded from radionuclide therapy.52 Despite these findings, there are still many gaps in our knowledge of delayed renal toxicity associated with 177Lu-PSMA-617, but ongoing trials are expected to provide further insights into the long-term renal safety profile of 177Lu-PSMA-617 therapy.
As 177Lu-PSMA-617 therapy moves into the early stage of disease, adaptive dosing is emerging as an important tool for delaying or reducing the radiation dose in patients who do not benefit from the treatment, thus reducing long-term toxicity. This approach was utilized in the ENZA-p trial where patients received adaptive-dosed 177Lu-PSMA-617 every 6–8 weeks depending on an interim PSMA-PET/CT.32
Conclusions
-
Radionuclide-based theranostics is ready for widespread adoption in prostate cancer therapy, including in early treatment lines.
-
PSMA radioligand therapy should be reserved for patients with lesions that demonstrate greater PSMA uptake than that observed in the liver. The use of PSMA-SUVmean and nomograms is not recommended for determining the eligibility for PSMA radioligand therapy and FDG-PET should be reserved for specific cases.
-
The newly developed PROMISE V2 criteria may be a valuable guide to optimal patient selection in the radioligand treatment of prostate cancer.
-
Post-treatment SPECT can be used to monitor patient responses over time and adjust the treatment in a personalized manner.
-
While grade 1–2 marrow toxicity is common with PSMA-targeted radionuclide therapy, composite biomarkers will help identify the cause of marrow compromise and guide future treatments.
-
Renal toxicity requires further evaluation in clinical trials, with long-term toxicity becoming more relevant as the treatment moves towards earlier stages in patients with longer life expectancy.
Conflict of interest
The authors have declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
The authors created and approved the final manuscript.


_mean____10_in_therap.jpeg)





_mean____10_in_therap.jpeg)

