Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, representing approximately 10% of all hematologic malignancies and 1% of all cancers.1 MM is characterized by the accumulation of clonal plasma cells within the bone marrow, which leads to disruption of normal hematopoiesis, and by the abnormal production of immunoglobulins, which can be detected in both urine and serum.2,3 At diagnosis, patients with MM often present with persistent but nonspecific symptoms, which may cause delays in diagnosis and treatment initiation. The most frequent symptoms include anemia-associated fatigue, bone pain due to lytic lesions or pathological fractures, edema due to renal failure and hypercalcemia-associated behavioral changes.4,5
Recent advances in MM treatment include immunomodulatory drugs (IMiDs), proteasome inhibitors (PI) and monoclonal antibodies (mAbs), used both as monotherapies and in combination regimens, and coupled with autologous stem cell transplantation (ASCT), as well as bispecific antibodies, immunoconjugates and chimeric antigen receptor (CAR) T-cell therapies.6–10 Despite these developments, MM remains an incurable hematological malignancy due to its extensive heterogeneity and ongoing clonal evolution, marked by repeated episodes of remission and relapse.
Updates in treatment of newly diagnosed multiple myeloma
IMROZ: Long-term PFS and OS benefits with isatuximab plus RVd in transplant-ineligible patients with NDMM
The transmembrane glycoprotein CD38, uniformly expressed on MM cells, functions as a receptor and ectoenzyme and regulates migration, signal transduction and receptor-mediated cell adhesion.11 Isatuximab is an IgG1 mAb that binds to a unique epitope on CD38, facilitating multimodal targeting and elimination of CD38-expressing MM cells through Fc-dependent immune effector mechanisms, such as complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). Isatuximab can directly induce apoptosis of CD38-expressing cells, inhibit CD38 ectoenzyme activity and modulate other immune cell populations.
In Switzerland, isatuximab is currently approved in combination with pomalidomide and dexamethasone for the treatment of adult patients with relapsed/refractory MM (RRMM) after at least two prior lines of therapy, including lenalidomide and a PI, as well as in combination with carfilzomib and dexamethasone after at least 1–3 prior lines of therapy.12 Isatuximab has been further investigated in combination with bortezomib, lenalidomide and dexamethasone (Isa-RVd) in several phase III trials. These include the GMMG-HD7 study that evaluated Isa-RVd versus RVd alone for induction and maintenance therapy in transplantation-eligible patients with newly diagnosed MM (NDMM)13; the BENEFIT trial that compared Isa-RVd with Isa-Rd in transplant-ineligible NDMM patients14,15; the IMROZ trial that investigated Isa-RVd versus RVd in transplant-ineligible patients with NDMM.16,17
In the global phase III IMROZ study, 446 transplant-ineligible patients with NDMM aged ≤80 years were randomized 3:2 to receive either Isa-RVd (n=265) or RVd (n=181).16,17 During the treatment initiation phase, patients were given four 6-week cycles of Isa-RVd or RVd, with bortezomib administered twice weekly. During the subsequent maintenance phase, patients were given 4-weekly cycles of either isatuximab, lenalidomide and dexamethasone (Isa-Rd) or lenalidomide and dexamethasone (Rd) until disease progression, unacceptable toxicity or patient withdrawal.
At a median follow-up of five years, the primary endpoint of progression-free survival (PFS) was met, with a statistically significant 40.4% reduction in the risk of progression or death with Isa-RVd versus RVd.16,17 The median PFS was not reached in the Isa-RVd arm compared with 54.3 months in the RVd arm, with 60-month PFS rates of 63.2% versus 45.2%, respectively (HR: 0.596 [98.5% CI: 0.406–0.876]; p=0.0005) (Figure 1). Subgroup analyses revealed PFS benefits with Isa-RVd across most patient subgroups, including some difficult-to-treat populations with negative prognostic factors, such as the presence of high-risk chromosomal abnormalities, Revised International Staging System (R-ISS) stage III disease and extramedullary disease at baseline. Although the overall survival (OS) data were still immature at a median follow-up of five years, there was a trend favoring Isa-RVd, with a 22.4% reduction in mortality risk in the Isa-RVd arm versus the RVd arm (OS rates, 72.3% vs 66.3%).
Safety analysis showed that the Isa-RVd regimen was overall well tolerated and the safety profile remained consistent with the known safety profiles for each agent.16,17 The rates of neutropenia and infections were higher with Isa-RVd than with RVd (grade ≥3, 54.4% vs 37.0% and 44.9% vs 38.1%, respectively). Infections in the quadruplet arm could be mitigated by prophylaxis.
Updated data demonstrated that Isa-RVd achieved significantly higher minimal residual disease (MRD) negativity rates and deeper responses compared with RVd.18 A significantly greater proportion of patients experienced sustained MRD negativity at a sensitivity of 10-5 for at least 12 months with Isa-RVd (46.8% vs 24.3% with RVd; p<0.0001). Additionally, 25.7% of patients in the Isa-RVd arm maintained MRD negativity for 36 months or longer compared with only 7.2% in the RVd arm. Achieving MRD negativity significantly reduced the risk of progression or death. Patients who achieved MRD negativity at any point had a 44% risk reduction with Isa-RVd (HR: 0.564 [95% CI: 0.325–0.979]; p=0.0418), and those achieving it within six months demonstrated a 42% reduction (HR: 0.582 [95% CI: 0.296–1.068]; p=0.0784).
The improved efficacy of Isa-RVd also translated into better health-related quality of life (HRQoL).19 Patients receiving Isa-RVd reported faster and more durable improvements in HRQoL compared with those receiving RVd, including physical functioning, pain and dyspnea, over a follow-up period of approximately five years. The authors concluded that the greater improvements in certain disease-related symptoms, such as QLQ-C30 pain and physical functioning, in the Isa-RVd arm may be due to deeper disease control achieved with this regimen.
BENEFIT: Adding bortezomib to isatuximab plus Rd is effective and safe in transplant-ineligible patients with NDMM
The Isa-RVd regimen was further compared with Isa-Rd in transplant-ineligible patients with NDMM in the randomized phase III BENEFIT (IFM 2020-05) study.14,15 Patients (median age, 73 years) underwent 1:1 randomization to receive either Isa-RVd (n=135) or Isa-Rd (n=135) for 12 4-week cycles, followed by either isatuximab, bortezomib and lenalidomide (Isa-VR) or isatuximab and lenalidomide (Isa-R) during cycles 13–18. From cycle 19, both arms received Isa-R until disease progression, unacceptable toxicities or consent withdrawal.
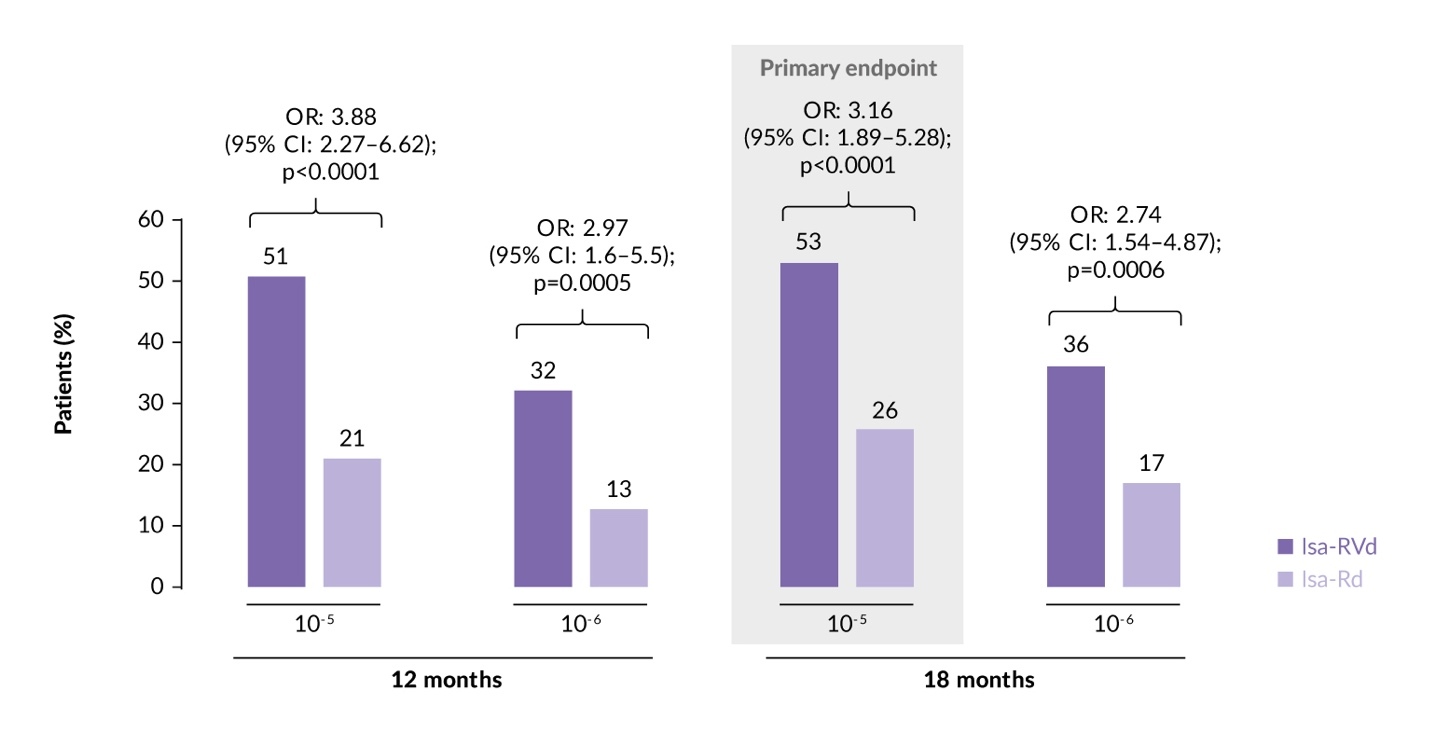
The study met its primary endpoint, demonstrating that treatment with Isa-RVd led to deeper responses and significantly higher MRD negativity rates at the 10-5 and 10-6 thresholds.14,15 Specifically, at 18 months, the MRD negativity rate (10-5 sensitivity) in the intention-to-treat (ITT) population was 53% with Isa-RVd versus 26% with Isa-Rd (odds ratio [OR]: 3.16 [95% CI: 1.89–5.28]; p<0.0001) (Figure 2). Subgroup analyses confirmed consistent MRD benefits of Isa-RVd over Isa-Rd across most subgroups, including those with negative prognostic factors, such as high-risk cytogenetics and R-ISS stage III disease. At a median follow-up of 23.5 months, the PFS and OS data remained immature. The estimated 24-month PFS rates were 85.2% with Isa-RVd versus 80.0% with Isa-Rd, while the estimated 24-month OS rates were 91.1% versus 91.5%, respectively.
In a recent analysis of patients with high-risk disease, high-risk features were identified in 32 (24%) and 24 (18%) patients in the Isa-RVd or Isa-Rd arms, respectively, based on the novel International Myeloma Society (IMS) definition of high-risk disease.20 At 18 months, the MRD negativity rate at 10-5 sensitivity was higher for Isa-RVd in high-risk patients, at 56% versus 25% with Isa-Rd (OR: 3.86 [95% CI: 1.2–12.3]). Similar MRD negativity trends were observed at the 10-6 threshold at 18 months (50% vs 25%; OR: 3.00 [95% CI: 0.95–9.52]). Higher MRD negativity rates were also observed at 12 months at both 10-5 and 10-6 sensitivity. Isa-RVd also demonstrated higher MRD negativity rates in patients achieving complete response or better (≥CR) status. In non-high-risk patients, Isa-RVd continued to show superior MRD negativity at 18 months both at 10-5 (OR: 3.00 [95% CI: 1.7–5.3]) and at 10-6 (OR: 2.61 [95% CI: 1.3–5.0]), as well as at 12 months, across MRD thresholds and response criteria. Although no significant interaction was observed between high-risk and non-high-risk groups, Isa-RVd consistently provided greater MRD negativity benefits for high-risk patients at all time points and thresholds and MRD negativity definition (≥CR and <CR).
In terms of safety, Isa-RVd was generally well tolerated, and the safety profile remained consistent with the known safety profiles of each constituent agent used in combination therapies.14,15 The rates of grade ≥3 treatment-related adverse events (TRAEs) were 69% in the Isa-RVd arm compared with 67% in the Isa-Rd arm. Together, these findings underscore the significant benefit of Isa-RVd over Isa-Rd in transplant-ineligible patients with NDMM, particularly those with high-risk features, demonstrating that the Isa-RVd regimen with weekly bortezomib is a feasible, effective and well-tolerated quadruplet option for fit older patients in this population.
GMMG-HD7: Effect of adding isatuximab to RVd induction and lenalidomide maintenance in transplantation-eligible patients with NDMM
The ongoing phase III GMMG-HD7 study aimed to evaluate Isa-RVd versus RVd for induction and maintenance therapy in transplantation-eligible patients with NDMM.21 In this trial, patients aged 18–70 years eligible for high-dose therapy (HDT) and ASCT (n=662) were randomly assigned in a 1:1 ratio to receive either Isa-RVd or RVd during the induction phase for three 6-week cycles prior to HDT and ASCT. Subsequently, patients were randomized 1:1 for maintenance therapy with isatuximab and lenalidomide or lenalidomide alone in 4-week cycles.
Results from the first part of this study demonstrated that adding isatuximab to RVd significantly improved MRD negativity rates versus RVd alone at the end of induction therapy (50.1% vs 35.6%; p<0.001).13,22 MRD negativity response deepened further, with post-transplant MRD negativity rates of 66.2% with Isa-RVd compared with 47.7% with RVd alone (p<0.0001).23 MRD negativity was also significantly higher among patients achieving CR (38.1% vs 25.8%; p=0.001) or a very good partial response or better (≥VGPR) (63.4% vs 43.8%; p<0.0001) in the Isa-RVd arm. Among patients who achieved CR, the MRD negativity rate post-transplant was 38.1% versus 25.8% (p=0.001) and among those with ≥VGPR, the MRD negativity rates were 63.4% and 43.8% (p<0.0001). The rates of continued MRD negativity, defined as MRD negativity persisting from post-induction to post-transplant, were also higher in the isatuximab-containing arm (53.1% vs 38.0%; p=0.0008).
The recently presented final PFS analysis further revealed a significant clinical benefit with Isa-RVd, regardless of the maintenance therapy strategy.24,25 In the ITT population (Isa-RVd, n=331; RVd, n=329), Isa-RVd induction without consolidation therapy reduced the risk of progression or death by 30% compared with RVd alone (HR: 0.70 [95% CI: 0.52–0.95]; p=0.0184) (Figure 3). At three and four years, the PFS rates were 83% and 76% for Isa-RVd versus 75% and 69% for RVd, respectively.
MRD negativity was a key predictor of improved PFS, with landmark analyses showing longer PFS in MRD-negative compared with MRD-positive patients (HR: 0.38 [95% CI: 0.26–0.55] post-induction; HR: 0.42 [95% CI: 0.28–0.63] post-transplant; both p<0.001).23,25 Treatment with Isa-RVd provided a PFS benefit over RVd in MRD-positive patients at the end of induction (HR: 0.64 [95% CI: 0.43–0.96]; p=0.03), but showed similar PFS outcomes for MRD-negative patients in the two treatment arms. Continued MRD negativity from post-induction to post-transplant was also associated with significantly longer PFS (HR: 0.41 [95% CI: 0.25–0.65]; p<0.001). Isa-RVd showed a trend toward longer PFS in patients without continued MRD negativity compared with RVd alone, although the difference was not statistically significant (HR: 0.68 [95% CI: 0.41–1.13]; p=0.14). Taken together, these results show that Isa-RVd significantly improved MRD negativity and provided PFS benefits, particularly in MRD-positive patients, despite the absence of consolidation therapy.
CASSIOPEIA: Long-term benefits of daratumumab plus VTd followed by daratumumab maintenance in transplant-eligible patients with NDMM
Daratumumab is a human anti-CD38 mAb that induces cell death in MM cells through multiple mechanisms, including CDC, ADCC, ADCP and apoptosis.26 In Switzerland, it is approved as monotherapy for MM patients who have received at least three prior lines of therapy, including a PI and an IMiD, or who are double-refractory to at least one PI and IMiD.27 In addition, daratumumab is approved in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone for patients with at least one prior line of therapy, and in combination with lenalidomide and dexamethasone or with bortezomib, melphalan and prednisone for previously untreated patients ineligible for ASCT.
The phase III CASSIOPEIA trial assessed daratumumab in combination with bortezomib, thalidomide and dexamethasone (D-VTd) in patients with transplant-eligible patients with NDMM.28,29 Patients were randomized 1:1 to receive either four cycles of D-VTd (n=543) or VTd alone (n=542) prior to transplantation, followed by two additional cycles of the same therapy post-transplant. Patients with a partial response or better were then randomized to either daratumumab monotherapy or observation for up to two years or until disease progression. At a median follow-up of 80.1 months from the first randomization, the median PFS was approximately 2.5 years longer with D-VTd than with VTd (83.7 months vs 52.8 months; HR: 0.61 [95% CI: 0.52–0.72]; p<0.0001).30,31 Furthermore, patients treated with D-VTd had a 45% reduced risk of death compared with those treated with VTd, with 72-month OS rates of 86.7% versus 77.7% (HR: 0.55 [95% CI: 0.42–0.73]; p<0.0001). At a median follow-up of 70.6 months from the second randomization, patients who received daratumumab experienced a 51% reduction in the risk of progression or death compared with patients who received observation, with median PFS not reached versus 45.8 months and 72-month PFS rates of 57.1% versus 36.5%, respectively (HR: 0.49 [95% CI: 0.40–0.59]; p<0.0001) (Figure 4). The longest PFS was observed in patients who received D-VTd followed by daratumumab maintenance. Importantly, in contrast to previous analyses that showed improved PFS with daratumumab maintenance only in patients who received VTd during induction and consolidation,32 this extended follow-up demonstrates that patients already exposed to daratumumab during induction also experience a benefit from daratumumab maintenance.30,31
MRD negativity rates at both 10-5 and 10-6 thresholds were higher for D-VTd followed by daratumumab (65.1% and 58.1%) compared with other regimens, including D-VTd followed by observation (58.1% and 48.9%), VTd followed by daratumumab (53.5% and 43.7%) and VTd followed by observation (36.3% and 26.5%).30,31 Sustained MRD negativity rates at ≥12 and ≥24 months were also the highest in patients treated with D-VTd followed by daratumumab.
In conclusion, this long-term follow-up of the CASSIOPEIA trial demonstrated sustained benefits of D-VTd induction therapy followed by daratumumab maintenance therapy in transplant-eligible patients with newly diagnosed MM, offering significant improvements in PFS, OS and MRD negativity rates.30,31
PERSEUS: Deepened responses with subcutaneous daratumumab plus RVd in transplant-eligible patients with NDMM
The subcutaneous formulation of daratumumab has proven to be non-inferior to the intravenous formulation in efficacy and safety, with fewer infusion-related reactions and a shorter administration duration.33 The phase III PERSEUS trial further assessed subcutaneous daratumumab combined with RVd induction and consolidation therapy and lenalidomide maintenance (D-RVd) compared with RVd with lenalidomide maintenance alone in transplant-eligible NDMM patients.34,35 A total of 709 patients were randomized 1:1 to receive either daratumumab plus RVd induction and consolidation followed by daratumumab plus lenalidomide maintenance (n=355) or RVd induction and consolidation and lenalidomide maintenance alone (n=354). The primary endpoint was PFS and the key secondary endpoints included rates of ≥CR and MRD negativity.
At a median follow-up of 47.5 months, the D-RVd regimen resulted in improved PFS versus RVd alone, with 48-month PFS rates of 84.3% versus 67.7%, respectively (HR: 0.42 [95% CI: 0.30–0.59]; p<0.001) (Figure 5).35 The D-RVd arm achieved deeper responses, with a ≥CR rate of 87.9% versus 70.1% in the RVd arm.34 The MRD negativity rate at 36 months (10-6 sensitivity) doubled with D-RVd compared with and sustained MRD negativity rates were 2.5 times higher with D-RVd versus RVd at both 12 months (47.3% vs 18.6%) and 18 months (42.0% vs 15.0%). Recently reported HRQoL outcomes demonstrated durable improvements in overall HRQoL, physical functioning and MM-related symptoms, such as pain and fatigue, with D-RVd.36 These improvements were comparable to those observed with RVd, indicating no adverse impact on HRQoL with the addition of daratumumab during induction, consolidation and maintenance phases.
Updates in treatment of relapsed/refractory multiple myeloma
DREAMM-7: Belantamab mafodotin as second-line therapy is effective and safe in patients with RRMM
As patients with MM frequently develop resistance or relapse on first-line triplet or quadruplet regimens, there is an urgent need for effective second-line combinations incorporating new therapy classes.37,38 Due to its highly selective expression on malignant plasma cells, B-cell maturation antigen (BCMA) is an established therapeutic target in MM.39 Belantamab mafodotin (belamaf) is a humanized anti-BCMA mAb conjugated to the microtubule inhibitor monomethyl auristatin-F via a protease-resistant cysteine linker.40,41 The global, randomized, open-label, phase III head-to-head DREAMM-7 trial evaluated belamaf, bortezomib and dexamethasone (BVd) versus daratumumab, bortezomib and dexamethasone (DVd) in patients with RRMM.42,43 Adult patients with at least one prior line of therapy and documented disease progression during or after their most recent therapy were randomized 1:1 to receive either BVd (n=243) or DVd (n=251) for eight cycles, followed by belamaf monotherapy or daratumumab monotherapy, respectively, from cycle 9 onward.
Treatment with BVd demonstrated a statistically significant and clinically meaningful PFS benefit, with a 23-month increase in median PFS compared with DVd.42,43 The median PFS was 36.6 months for BVd versus 13.4 months for DVd, with 18-month PFS rates of 69% versus 43%, respectively (HR: 0.41 [95% CI: 0.31–0.53]; p<0.00001). Pre-specified subgroup analyses showed consistent PFS benefits with BVd, including patients refractory to lenalidomide treatment or those with high-risk cytogenic features. BVd also provided long-term clinical benefits, as reflected in the time to disease progression or death from any cause after subsequent anti-myeloma therapy (PFS2) (not reached vs 33.4 months; HR: 0.59 [95% CI: 0.45–0.77]).44
Updated data revealed significant OS improvements with BVd over DVd.44 At a median follow-up of 39.4 months, the median OS was not reached in either treatment arm (HR: 0.58 [95% CI: 0.43–0.79]; p=0.00023) (Figure 6). Modeled projections estimated a median OS of 84 months for BVd compared with 51 months for DVd. The estimated 24-month survival rates were 79% with BVd and 67% with DVd, while 36-month OS rates were 74% and 60%, respectively.
BVd also yielded deeper responses compared with DVd, with an objective response rate (ORR) of 83.1% versus 71.3% and ≥CR rates of 35.8% versus 17.5%, respectively, at the latest update.44 MRD negativity rates (at 10-5 sensitivity) were more than doubled in the BVd arm, at 25.1% versus 10.4% in patients achieving ≥CR, and 38.7% versus 17.9% in those achieving ≥VGPR (p<0.00001).
The safety and tolerability of BVd were consistent with the known safety profiles of the individual agents, and side effects were manageable with dose delays.42,43 Exposure-adjusted infection rates per 100 person-years were comparable between the BVd and DVd arms for all grades (51.1 vs 55.4), but slightly higher for grade ≥3 infections with BVd (22.5 vs 16.4). All-grade ocular AEs were more common with BVd versus DVd (79% vs 29%). Among patients who received BVd, 44% required dose reductions, 78% had dose delays/interruptions (which did not negatively impact PFS) and 9% discontinued treatment due to ocular AEs. Despite these safety considerations, no difference in global quality of life (QoL) was observed between the treatment arms. Based on the presented data, BVd could become an option for second-line treatment of MM. However, alternative treatment options with different safety profiles are available and ongoing studies evaluating BCMA-targeting bispecific antibodies and BCMA-directed CAR T cells in early lines of therapy may challenge the targeting of BCMA by belantamab mafodotin.
DREAMM-8: Belantamab mafodotin plus pomalidomide and dexamethasone in RRMM
Belamaf was further evaluated in RRMM in the phase III DREAMM-8 trial that compared its combination with pomalidomide and dexamethasone (Pd) versus pomalidomide plus Vd (PVd) in patients previously treated with at least one line of therapy, including lenalidomide.45 This open-label study enrolled 302 adult patients with disease progression during or after their most recent therapy, who were randomized 1:1 to receive Belamaf-Pd (n=155) or PVd (n=147). The primary endpoint was PFS and the key secondary endpoints included OS, MRD negativity and duration of response (DoR).
At a median follow-up of 21.8 months, treatment with Belamaf-Pd resulted in a 48% reduction in the risk of progression of death versus PVd (HR: 0.52 [95% CI: 0.37−0.73]; p<0.001), with the median PFS not reached versus 12.7 months and 12-month PFS rates of 71% versus 51%.45 The benefit was consistent across all subgroups, including patients refractory to lenalidomide and anti-CD38 therapy. Belamaf-Pd regimen resulted in higher rates of ≥CR (40% of patients vs 16% in the PVd arm) and MRD negativity (24% vs 5% with PVd in patients with ≥CR), as well as improved DoR (median, not reached vs 17.5 months; 12-month DoR rate, 79% vs 61% with PVd). There was an OS trend in favor of Belamaf-Pd (median, not reached in either treatment arm; 12-month OS rate, 83% vs 76%; HR: 0.77 [95% CI: 0.53−1.14]).
Regarding safety, grade ≥3 AEs were reported in 94% of patients receiving Belamaf-Pd compared with 76% receiving PVd.45 The incidence of treatment discontinuations or dose reductions was similar between the treatment arms. As in DREAMM-7, ocular events were frequent with Belamaf-Pd, occurring in 89% of patients compared with 30% in the PVd arm (grade 3–4, 43% vs 2%). Ocular AEs in the Belamaf-Pd arm were managed with dose modifications, resulting in a treatment discontinuation rate of 9%.
In summary, these data demonstrate significant benefits of Belamaf-Pd with respect to PFS and response to treatment in lenalidomide-exposed RRMM patients, with ocular AEs being generally reversible and manageable.45
CAMMA 2: Promising efficacy with cevostamab in triple-class refractory MM
The cell surface protein FC-receptor homolog 5 (FcRH5), also known as CD307, is exclusively expressed in the B-cell lineage and is more strongly expressed by myeloma cells than by normal B cells, making it a promising new therapeutic target for MM.46,47 Cevostamab is an IgG1-based T cell-engaging bispecific antibody that targets the membrane-proximal domain of FcRH5 on myeloma cells and the epsilon domain of CD3 on T cells.46
In a phase I dose-finding study, cevostamab demonstrated promising activity in patients with heavily pretreated RRMM, including those previously exposed to BCMA-targeted agents.48 Cohort A1 of the multicohort phase I/II CAMMA 2 study evaluated the efficacy and safety of cevostamab in patients with triple-class refractory RRMM and prior exposure to either BCMA-targeted antibody-drug conjugate (ADC, n=10) or CAR T-cell therapy (n=11). During the first 21-day treatment cycle, 21 patients (median age, 64 years) received escalating doses of cevostamab (0.3–160 mg), followed by fixed 160 mg dosing in cycles 2 and 3.49 Cohort A2 enrolled patients with triple-class refractory MM who had received prior BCMA-targeted therapies, including at least one BCMA-targeted bispecific antibody.50
AEs were observed in all treated Cohort A1 patients, with a safety profile consistent with prior data on cevostamab and no relevant off-target effects.49 Cytokine release syndrome (CRS) was reported in 71% of patients in the ITT population (grade 2, 48%), 90% of those previously treated with ADC (grade 2, 50%) and 55% of those previously treated with CAR T-cell therapy (grade 2, 46%). CRS occurred primarily during the first treatment cycle, with a median duration of one day, and was effectively managed with corticosteroids and/or tocilizumab. Grade 3 infections were reported in 24% of patients, with no grade 4 infections, which is favorable compared with BCMA-targeting bispecifics.
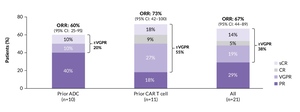
At a median follow-up of 11 months, six of 14 responders maintained their response.49 The ORR was 67%, with 38% of patients achieving ≥VGPR (Figure 7). By prior treatment, the ORR was 60% among patients previously treated with ADC (≥VGPR, 20%) and 73% among those previously treated with CAR T-cell therapy (≥VGPR, 55%). Notably, recently presented results from Cohort A2 showed that clinical responses were less frequent in the prior bispecific antibody group than in the prior ADC and CAR T-cell therapy groups.50 Objective responses were observed in two of 21 patients (10%), including one CR and one partial response. Ten patients (48%) had stable disease. The authors concluded that the lower frequency of responses in this subset may be due the higher number of prior lines of therapy and T-cell exhaustion due to multiple prior exposures to bispecific antibodies. These results suggest that cevostamab is highly active in patients with triple-class refractory RRMM and a prior exposure to either BCMA-targeted ADC or CAR T-cell therapy, demonstrating its potential role in the management of this patient population.
Conclusion
Recent updates in MM management have highlighted important developments in treatment strategies, with improved outcomes for many patients. Key advances include the introduction and refinement of monoclonal and bispecific antibodies used in combination regimens. Trials involving drugs such as isatuximab, daratumumab, belantamab mafodotin and cevostamab have demonstrated improved survival outcomes, higher MRD negativity rates and better overall responses, even in challenging patient populations. These innovations not only extend survival but also improve patients’ QoL. The progress in MM treatments underscores a promising trajectory toward more effective management of this complex hematologic malignancy.
Conflict of interest
Dr Rouven Müller received honoraria for consulting and advisory boards from AbbVie, Amgen, BMS, GSK, Johnson & Johnson, Pfizer and Sanofi. These funding entities and sponsors did not play a role in the development of the manuscript and did not influence its content in any way.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_analysis_of_the_imroz_trial.jpg)

_from_the_first_randomization_in_the_gmmg-hd7_trial.jpeg)
_and_overall_survival_(os)_after_first_randomization_in_the.jpeg)
_in_the_perseus_trial.jpeg)
_significantly_prolonged_overa.jpeg)
_analysis_of_the_imroz_trial.jpg)
_from_the_first_randomization_in_the_gmmg-hd7_trial.jpeg)
_and_overall_survival_(os)_after_first_randomization_in_the.jpeg)
_in_the_perseus_trial.jpeg)
_significantly_prolonged_overa.jpeg)
