Introduction
Endocrine therapy is the first-line therapy for hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer, with robust clinical evidence supporting its efficacy. In this patient population, randomized phase III trials have demonstrated significant antitumor activity of third-generation aromatase inhibitors, such as letrozole and anastrozole, as well as selective estrogen receptor degrader (SERD) fulvestrant, with a median progression-free survival (PFS) ranging from 10 to 16 months.1,2 However, endocrine resistance, which can be either de novo or acquired, remains a significant challenge. Numerous mechanisms and pathways contribute to this resistance, prompting the development of targeted therapeutic agents designed to overcome these barriers. Available treatment options include cyclin-dependent kinase 4/6 (CDK4/6) inhibitors such as abemaciclib, ribociclib and palbociclib,3–5 the AKT inhibitor capivasertib,6 phosphatidylinositol 3-kinase (PI3K) inhibitors such as alpelisib7,8 and inavolisib,9 poly (ADP-ribose) polymerase (PARP) inhibitors such as olaparib10 and talazoparib,11 oral SERDs such as elacestrant,12 as well as antibody-drug conjugates (ADCs) sacituzumab govitecan (SG) and trastuzumab deruxtecan (T-DXd) (in HER2-low and ultra-low disease).13–16
For patients with HR-positive, HER2-negative advanced breast cancer, the combination of an aromatase inhibitor with a CDK4/6 inhibitor is the standard of care, demonstrating improved survival outcomes compared with aromatase inhibitor therapy alone.17 Although initial treatment yields benefits, most patients eventually face disease progression, making the selection of subsequent therapeutic options a complex clinical challenge.18,19 Essentially a protein kinase, with three isoforms, each involved in cellular survival, glucose metabolism and neuronal growth, AKT is the key component of the PI3K/AKT/PTEN signaling pathway, which is overactivated in approximately 50% of HR-positive, HER2-negative breast cancers due to activating mutations in PIK3CA and AKT1 and loss of PTEN function.20 In the phase III CAPItello-291 trial, capivasertib, an orally bioavailable, small-molecule inhibitor of all three AKT isoforms,21 significantly prolonged PFS in combination with fulvestrant, when compared with placebo plus fulvestrant in patients previously treated with a CDK4/6 inhibitor.6 Safety data from the trial indicated that the most frequent grade 3 or higher adverse events associated with capivasertib plus fulvestrant were rash (12.1% vs 0.3% with placebo plus fulvestrant), diarrhea (9.3% vs 0.3%) and hyperglycemia (2.3% vs 0.3%).
We present the clinical case of a patient with HR-positive, HER2-negative breast cancer who achieved clinical benefit from capivasertib after disease progression on a range of therapies, including ovarian suppression, aromatase inhibitors, fulvestrant, chemotherapy and the combination of palbociclib with letrozole.
Case presentation
In 2006, a 45-year-old patient presented with a fine nodular finding on palpation of the left breast. Mammography was inconclusive for disease. The biopsy sample revealed invasive ductal carcinoma, for which the patient underwent lumpectomy with sentinel lymph node biopsy and axillary revision. She was diagnosed with multicentric invasive ductal HR-positive, moderately differentiated, HER2-negative breast cancer, classified as stage IB with micrometastases in one out of 15 examined regional lymph nodes, with clear surgical margins (pT1c, pN1mi (1/15), G2, R0). At this point, bone scintigraphy was ordered to rule out osseous metastases, but no other staging was performed. Given the favorable tumor biology, limited nodal involvement and tumor confinement to a single breast region, mastectomy was not considered necessary.
Following an interdisciplinary tumor board recommendation, the patient underwent adjuvant radiotherapy and endocrine therapy, including ovarian function suppression with goserelin, and tamoxifen for two years, followed by three years of aromatase inhibitor therapy.
In 2012, the patient developed retromamillary local recurrence. Radiographic investigations using liver ultrasound and chest X-ray revealed no other suspicious findings. Based on international guidelines, simple mastectomy of the affected breast was recommended. She subsequently underwent unilateral mastectomy, followed by endocrine therapy with letrozole.
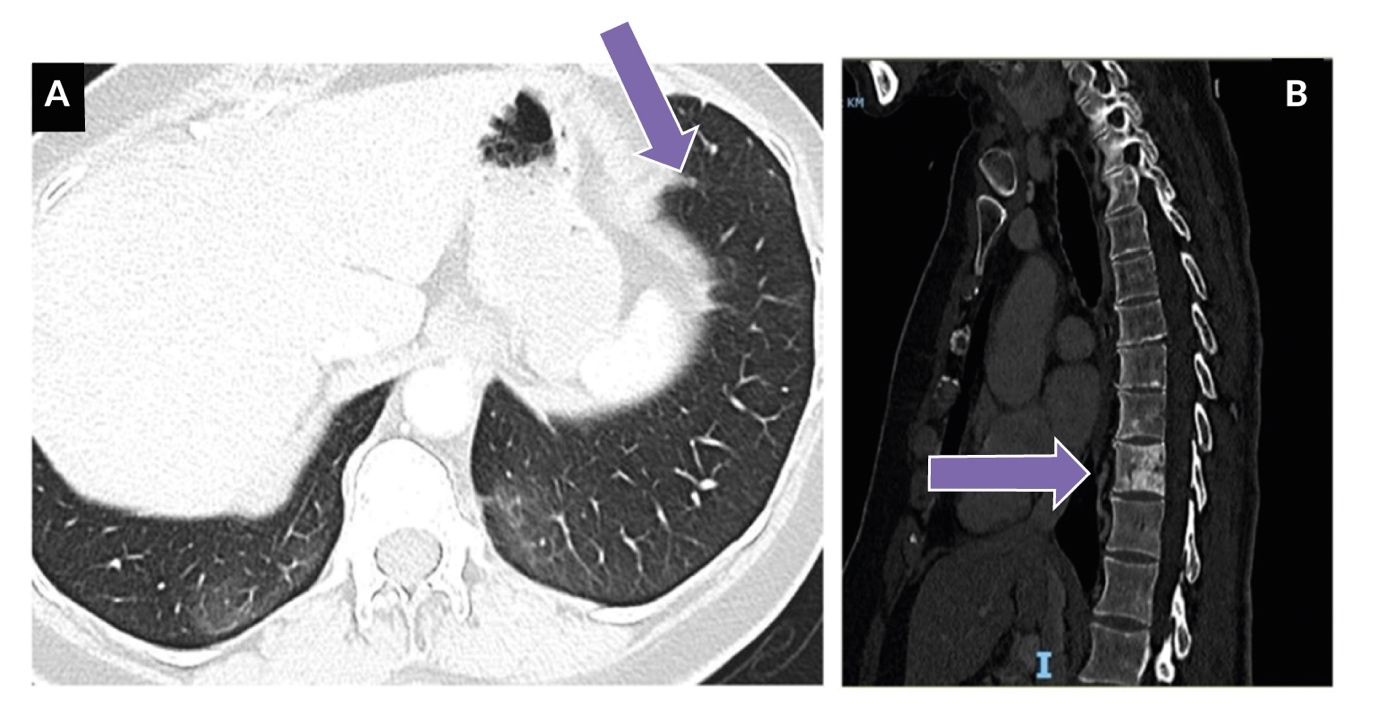
Surgical resection revealed a 2.3 cm lesion with skin infiltration and lymphangitis carcinomatosis. Histopathological examination confirmed moderately differentiated invasive ductal breast carcinoma with 100% estrogen and progesterone receptor positivity on immunohistochemistry and an MIB-1 proliferation index of 20%. By the end of 2012, the patient again experienced local relapse and two additional tumor-infiltrating nodular skin lesions were resected. Given the high-risk nature of the recurrence, the patient’s prior endocrine therapy with letrozole was deemed insufficient to prevent further progression. Therefore, a clinical decision was made to switch to fulvestrant (500 mg/month), despite it being outside standard adjuvant guidelines, due to its efficacy in endocrine-resistant disease and the need for a more potent estrogen receptor blockade to reduce the risk of further recurrence. The patient tolerated fulvestrant well and maintained stable disease from late 2012 to 2016, with no further local recurrences or evidence of systemic progression in radiographic investigations (bone scintigraphy, ultrasound and mammography and chest X-ray) during this period. In 2016, due to a local injection reaction, the patient transitioned to tamoxifen (20 mg/day). Unfortunately, in the summer of 2017, the patient developed visceral metastases, presenting with a lung nodule in the lingula, a right hilar tumor lesion infiltrating the bronchial wall and right axillary nodal metastases. Notably, no bone metastases were detected at this time (Figure 1).
The patient received radiotherapy targeting the right hilar metastasis and osteoprotective therapy with denosumab was initiated. At this point, the patient remained on systemic treatment with tamoxifen. In the spring of 2018, a new metastasis was identified on the chest wall and an excisional biopsy was performed. DNA next-generation sequencing (NGS) of the tumor tissue revealed a somatic BRCA2 mutation in exon 20 (p.E2877*), BRCA1 mutation in exon 2 (p.E10K) and two PIK3CA mutations in exon 21 (p.H1047R) and exon 2 (p.R108C). Given the identification of BRCA mutations, the patient was referred for genetic counseling and offered germline genetic testing; however, she declined, noting that she had no sisters and was the mother of a son.
Furthermore, restaging computed tomography (CT) performed after the excision showed slight progression of both lymphadenopathy and pulmonary metastases, prompting the necessity to change therapy.
According to the international oncologic guidelines in place at the time, the patient was initiated on combination therapy with the CDK4/6 inhibitor palbociclib and letrozole. This regimen had a good initial therapeutic effect with resolution of lung metastases, demonstrating significant therapeutic efficacy. However, the treatment was complicated by adverse effects, including mood swings and joint pain attributed to letrozole. Furthermore, due to skin toxicity, the palbociclib dose was reduced from 125 mg to 100 mg daily.
The disease remained stable until a follow-up CT scan performed in November 2019 revealed modest disease progression, characterized by an increase in the size of two lung parenchymal lesions and one subpleural lesion. A subsequent radiographic evaluation one month later indicated further disease progression, with enlargement of lymph node metastases and the emergence of new bone metastases (Figure 2).
The patient was transitioned to oral chemotherapy with capecitabine at a dose of 1,000 mg daily.
At this stage, treatment with alpelisib was not pursued despite the presence of PIK3CA mutations, which could have made the patient a candidate for this targeted therapy. While alpelisib was Food and Drug Administration (FDA)-approved in 2019, it did not receive regulatory approval in Switzerland until March 2020, and access to the drug may have been a limiting factor at the time of treatment decisions. Furthermore, since the patient had already received fulvestrant earlier in her treatment course, this may have influenced the decision to explore alternative therapeutic options rather than reintroducing a fulvestrant-based regimen.
However, after six months of therapy, radiographic evaluations indicated stable disease with partial regression of metastatic lesions. The patient remained on capecitabine for a total of four years. In 2024, the dose was increased to 2,000 mg daily due to a progressive rise in the CA 15–3 tumor marker, which increased from 43 kU/L in June 2020 to 68 kU/L in November 2023. During this period, bone disease remained stable and visceral metastases demonstrated an excellent therapeutic response.
By early 2024, the patient had reported treatment-related adverse events, including grade 2 hand-foot syndrome, diarrhea, joint pain with swelling and inappetence. Laboratory tests revealed hypercalcemia (2.7 mmol/L), which had likely persisted for approximately three months, alongside an elevated CA 15–3 level of 89 kU/L. At this time, fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT imaging demonstrated multiple sites of metabolically active and vital bone metastases (Figure 3).
In the spring of 2024, a liquid biopsy was performed to evaluate circulating tumor DNA (ctDNA) for tumor heterogeneity, verify estrogen receptor 1 (ESR1) mutation and monitor disease progression non-invasively. Using NGS with the Oncomine Pan-Cancer Cell-Free Assay (cfPanC), a PIK3CA mutation in exon 21 (p.H1047R) was detected at a variant allele frequency (VAF) of 0.85% (sensitivity: 0.16%). No mutations in BRCA1, BRCA2 or ESR1 were identified. This finding contrasts with the results from the 2018 tumor biopsy of the chest wall lesion, which revealed somatic mutations in both BRCA1 and BRCA2. This discrepancy likely reflects tumor heterogeneity, with the BRCA1/2-mutant subclone being either absent or minimally represented in ctDNA at the time of liquid biopsy.
At the time of liquid biopsy, the patient demonstrated good clinical status, with an Eastern Cooperative Oncology Group (ECOG) performance score of 0–1 and no significant comorbidities. Glycemic control was maintained, with an HbA1c level below 8%.
By the summer of 2024, the patient transitioned to treatment with capivasertib in combination with fulvestrant, at an initial dose of 800 mg daily, reduced to 600 mg daily due to grade 3 diarrhea. Thereafter, calcium levels normalized and the CA 15–3 tumor marker decreased to 40 kU/L, indicating a favorable therapeutic response. As of January 2025, the patient continues capivasertib therapy but recently reduced the dose to 400 mg daily due to ongoing grade 2 diarrhea. However, a moderate rise in the tumor marker CA 15–3 from 41.1 kU/L in December 2024 to 50 kU/L in early 2025 led to the recommendation to increase the dose back to 600 mg daily to maximize therapeutic efficacy. Blood glucose levels remained within the normal range and no additional adverse events were reported. Notably, since tumor markers decreased and remained stable, no additional imaging was necessary after the initiation of capivasertib in May 2024.
Discussion
This case presents the clinical course of a patient with HR-positive, HER2-negative breast cancer who achieved clinical benefit with capivasertib following disease progression after multiple lines of prior therapy. The patient initially responded to ovarian suppression and tamoxifen, followed by treatment with an aromatase inhibitor, with disease control of approximately four years. However, subsequent recurrence underscored the ongoing challenge of managing HR-positive disease in the long term.
The switch to the CDK4/6 inhibitor palbociclib was supported by data from the PALOMA-2 trial, which demonstrated significantly longer PFS compared with letrozole alone in this patient population.3 Unfortunately, the patient experienced skin toxicity, which can occur in approximately one-third of patients treated with a CDK4/6 inhibitor. In addition, despite effective control of visceral disease, the emergence of bone metastases aligned with supporting the routine clinical experience, which suggested that bone lesions may be less responsive to CDK4/6 inhibition.
The median PFS on the first-line CDK4/6 inhibitor ranges between two and three years,3–5 and most patients eventually develop resistance and require a change in therapy.18,19 For these patients, the optimal next line of therapy remains uncertain, with potential options including chemotherapy, fulvestrant and targeted therapies such as ADCs.17 Data have shown that capecitabine remains effective after progression on treatment with a CDK4/6 inhibitor plus endocrine therapy, regardless of treatment line and metastatic site.22 In our patient, the switch to capecitabine provided disease control for four years, consistent with expected treatment durations. The emergence of bone metastases and hypercalcemia required further treatment modifications due to ongoing disease activity despite systemic therapy.
Molecular testing confirmed the presence of a PIK3CA mutation in our patient, which led to the initiation of capivasertib in combination with fulvestrant. This approach was supported by the results from the CAPitello-291 trial, which showed improved PFS in patients with PIK3CA-altered tumors, with a median of 7.2 months compared with 3.6 months with placebo plus fulvestrant.6 Favorable survival outcomes with capivasertib plus fulvestrant were also reported in the phase II FAKTION trial on HR-positive, HER2-negative breast cancer without a prior CDK4/6 inhibitor.23,24 This combination regimen significantly prolonged PFS in both the overall population (median, 10.3 months vs 4.8 months with placebo plus fulvestrant) and the expanded pathway-altered subgroup (median, 12.8 months vs 4.6 months).24 These findings underline the importance of optimizing treatment sequencing rather than simply combining agents.
In two clinical trials, capivasertib was associated with grade 3 or higher adverse events, including rash, diarrhea and hyperglycemia.6,23 Our patient experienced grade 3 diarrhea, which was successfully managed with dose reductions.
Regarding future therapeutic regimens, capivasertib is currently being investigated in combination with a CDK4/6 inhibitor (palbociclib or ribociclib) and fulvestrant in patients with HR-positive, HER2-negative advanced breast cancer following recurrence or progression on or within 12 months of the end of (neo)adjuvant endocrine therapy in the phase Ib/III CAPItello-292 study.25 Other emerging promising therapies under evaluation include the PI3K inhibitor inavolisib, the oral SERD elacestrant and the PARP inhibitor olaparib. In the phase III INAVO120 trial, inavolisib in combination with palbociclib and fulvestrant significantly improved PFS, with a median of 15.0 months compared with 7.3 months with palbociclib plus fulvestrant alone in treatment-naïve patients with a HR-positive, HER2-negative metastatic breast cancer harboring a PIK3CA mutation who had had relapse during or within 12 months after the completion of adjuvant endocrine therapy.9 Improved PFS was also observed in the phase III EMERALD trial, with a 6-month PFS rate of 34.3% for elacestrant versus 20.4% for standard therapy in the overall population and 40.8% versus 19.1% in patients with ESR1 mutation.12 The phase III OlympiAD trial reported favorable PFS outcomes (7.0 months vs 4.2 months) for olaparib compared with chemotherapy in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer, including those with HR-positive disease.10 Finally, ADCs represent an effective treatment strategy in this setting. A trophoblast cell-surface antigen 2 (Trop-2)-directed ADC SG significantly improved overall survival (OS) versus chemotherapy (14.4 months vs 11.2 months) in HR-positive, HER2-negative metastatic breast cancer in the phase III TROPiCS-02 trial.16 HER2-targeting ADC T-DXd demonstrated activity not only in HER2-positive advanced breast cancer, but also in tumors with low and ultra-low HER2 expression. In the phase III DESTINY-Breast04 study, the median PFS in the HR-positive, HER2-low cohort was 9.6 months versus 4.2 months and the median OS was 23.9 months versus 17.6 months with T-DXd versus chemotherapy, respectively.15 In the phase III DESTINY-Breast06 study, T-DXd improved PFS compared with chemotherapy (13.2 months vs 8.1 months) in patients with HR-positive disease with HER2-low and HER2-ultralow expression levels.14
Conclusion
This case highlights the critical importance of individualized treatment strategies in HR-positive, HER2-negative metastatic breast cancer, particularly in addressing endocrine resistance and disease progression. Molecular profiling, especially PIK3CA mutation testing, is pivotal in guiding therapeutic decisions. Testing for PIK3CA mutations at the time of disease progression on endocrine and CDK4/6 inhibitors can identify patients who may benefit from targeted therapies such as capivasertib or PI3K inhibitors such as alpelisib. These mutations can be detected through the analysis of original tumor tissue or liquid biopsies, with the latter offering the advantage of identifying dynamic genetic changes in real time. Serial liquid biopsies provide additional insight into tumor heterogeneity and evolving resistance mechanisms, enabling clinicians to tailor subsequent therapies to the patient’s disease biology.
For patients harboring PIK3CA mutations, targeted therapies such as capivasertib and PI3K inhibitors such as alpelisib have demonstrated efficacy in overcoming resistance mediated by the PI3K/AKT/PTEN pathway. Moreover, inavolisib, when combined with endocrine therapy and CDK4/6 inhibitors, has shown promising results in patients with PIK3CA-mutated disease, further expanding therapeutic options. These advancements underscore the necessity of carefully sequencing therapies to maximize clinical benefit. Determining the optimal order of endocrine therapies, CDK4/6 inhibitors, chemotherapy and PI3K/AKT inhibitors remains a pressing challenge in metastatic breast cancer management.
Clinical insight
Routine PIK3CA mutation testing at disease progression on endocrine and CDK4/6 inhibitor therapy is essential to guide personalized treatment strategies. Liquid biopsies are particularly valuable for monitoring tumor heterogeneity and detecting resistance mutations, such as AKT and ESR1, which can influence subsequent therapy. The expanding treatment landscape, which includes agents such as alpelisib, inavolisib and capivasertib, highlights the importance of thoughtful therapy sequencing to optimize outcomes, balance efficacy and minimize resistance.
Ethics approval and consent to participate
Ethics approval was not required for the study. Written consent for the further use of patient data was obtained.
Consent for publication
Consent for publication was obtained.
Availability of data and materials
All patient data that support this case report are included in the anonymized form in the published article.
Conflict of interest
Marcus Vetter received honoraria for consultancy from GSK, Roche, Novartis, ExactSciences, Pfizer, Stemline, AbbVie and ASC Oncology. Diana Chiru declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
All authors contributed to and approved the final manuscript.
_thorax_demonstrating_a_metastatic_tumor_lesion_in_the_ri.jpeg)


_thorax_demonstrating_a_metastatic_tumor_lesion_in_the_ri.jpeg)