AMPLIFY: Fixed-duration venetoclax plus acalabrutinib in untreated CLL
The AMPLIFY trial was one of the key highlights of the meeting.1,2 For the first time, a regimen combining fixed-duration venetoclax with the second-generation Bruton tyrosine kinase inhibitor (BTKi) acalabrutinib was investigated in previously untreated patients with chronic lymphocytic leukemia (CLL). Currently, treatment-naïve CLL patients have several treatment options, including continuous BTKi therapy or fixed-duration venetoclax regimens, both with or without anti-CD20 monoclonal antibody (mAb) obinutuzumab.3 While the combination of fixed-duration venetoclax with ibrutinib can achieve deep durable responses, it is associated with cardiac toxicity, particularly in older patients.4–8 Acalabrutinib is a highly selective BTKi characterized with improved safety profile compared with ibrutinib.9–11 AMPLIFY aimed to evaluate the efficacy and safety of fixed-duration venetoclax plus acalabrutinib with or without obinutuzumab versus the investigator’s choice of chemoimmunotherapy in fit, treatment-naïve patients with CLL without del(17p) or a TP53 mutation.1,2
This study followed a three-arm design.1,2 A total of 867 patients aged ≥18 years were randomized 1:1:1 to receive 14 cycles of acalabrutinib plus venetoclax (AV), 14 cycles of AV plus obinutuzumab (AVO) or six cycles of chemoimmunotherapy with fludarabine-cyclophosphamide-rituximab (FCR) or bendamustine-rituximab (BR), depending on the patient’s age and comorbidities. Patients with del(17p) or TP53 mutations, significant cardiovascular disease and a Cumulative Illness Rating Scale (CIRS)-Geriatric index >6 were excluded from the study. The primary endpoint was progression-free survival (PFS) of AV versus FCR/BR in the intent-to-treat (ITT) population. The secondary endpoints included PFS for AVO versus FCR/BR, undetectable measurable residual disease (uMRD) for AV and AVO versus FCR/BR and overall survival (OS) for AV and AVO versus FCR/BR.
The combination of acalabrutinib plus venetoclax met the primary endpoint, with significant PFS improvement compared with chemoimmunotherapy.1,2 At a median follow-up of 40.8 months, the median PFS was not reached in the AV arm compared with 47.6 months with FCR/BR (HR: 0.65 [95% CI: 0.49–0.87]; p=0.0038) (Figure 1). Furthermore, PFS was not reached and improved in the AVO arm versus FCR/BR as well (HR: 0.42 [95% CI: 0.30–0.59]; p<0.0001). The 36-month PFS rates in the AV, AVO and FCR/BR arms were 76.5%, 83.1% and 66.5%, respectively.
The secondary endpoint of OS was prolonged with the AV regimen versus FCR/BR (HR: 0.33 [95% CI: 0.18–0.56]; p<0.0001), with 36-month OS rates of 94.1% versus 85.9%, respectively.1,2 Adding obinutuzumab in the second arm also proved very effective; however, based on prior experience, patients treated with obinutuzumab during the COVID-19 pandemic generally had worse outcomes. This was also reflected in the AMPLIFY trial, where the majority of COVID-19-related deaths occurred in the obinutuzumab-containing arm. When censored for COVID-19-related deaths, the secondary endpoint of OS was improved both with the doublet (HR: 0.27 [95% CI: 0.11–0.60]) and the triple regimen (HR: 0.47 [95% CI: 0.22–0.95]) versus chemoimmunotherapy.
The highest uMRD rates at the end of treatment (EoT) were observed in the AVO arm (95.0% vs 45.0% with AV and 72.9% with FCR/BR).1,2 PFS was prolonged in patients who achieved uMRD at EoT in all three treatment arms, with PFS rates ranging from 74.8% with FCR/BR to 87.1% with AV and 90.4% with AVO.
With respect to safety, grade ≥3 adverse events (AEs) were reported in 53.6%, 69.4% and 60.6% of patients in the AV, AVO and FCR/BR arms, respectively.1,2 The most common AEs in the experimental arms included hematological toxicity and infections. Slightly higher rates of cardiac events were observed in both experimental arms (AV, 9.3%; AVO, 12%; FCR/BR, 3.5%), as well as more frequent hemorrhages. However, grade ≥3 AEs were manageable and did not raise significant concerns. The overall incidence of atrial fibrillation was low.
In conclusion, this study demonstrated good efficacy and manageable safety profile with AV versus FCR/BR, with similar outcomes for AVO versus FCR/BR.1,2 A key advantage of the AV regimen is its fixed-duration therapy, which is advantageous for patients and combines the two most effective drugs for this disease entity. While some patients may prefer monotherapy based on individual needs, this combination sets a new standard of care (SoC) and is a practice-changing approach for treatment-naïve CLL. Patients with unmutated IGHV-status may profit most from the addition of obinutuzumab to AV (AVO), but higher vulnerability against COVID-19-infection and grade 3 infections in general should be taken into account.
TRIANGLE: Ibrutinib with and without ASCT in MCL
The randomized TRIANGLE trial previously demonstrated high efficacy of adding ibrutinib during induction chemoimmunotherapy and as a maintenance therapy with and without autologous stem cell transplantation (ASCT) in younger patients with mantle cell lymphoma (MCL).12,13 The latest data presented at ASH 2024 provided further insights into the efficacy comparison of the ibrutinib-containing regimens with and without ASCT with prolonged follow-up of 55 months.14
The study enrolled patients aged ≤65 years with previously untreated, advanced-stage (II-IV) MCL eligible for high-dose cytarabine and ASCT.14 Patients (n=870) were randomized 1:1:1 into three arms, all receiving six alternating cycles of chemoimmunotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) and rituximab, dexamethasone, cytarabine and cisplatin (R-DHAP). In Arm A (control, n=288), chemoimmunotherapy was followed by ASCT. In the experimental arms, ibrutinib was added to chemoimmunotherapy followed by two years of ibrutinib maintenance with (Arm A+I, n=292) or without ASCT (Arm I, n=290). Rituximab maintenance was added following national guidelines. The primary endpoint was failure-free survival (FFS). The secondary outcomes included response rates, PFS, OS and safety.
The study demonstrated the ongoing superiority of the ibrutinib-containing regimen plus ASCT versus control (HR for A+I vs A: 0.64; p=0.0026), with the median FFS not reached in either arm and 4-year FFS rates of 82% versus 70%, respectively.14 The control regimen was not superior to ibrutinib-containing regimen without ASCT (HR for A vs I: 1.29; p=0.9890). In contrast, the retrospectively calculated two-sided p-value demonstrated FFS superiority of I over A (p=0.0208). When comparing the efficacy of the two ibrutinib-containing regimens, the addition of ASCT failed to show FFS superiority (HR for A+I vs I: 0.83; p=0.21; 4-year FFS rates: 82% vs 81%) (Figure 2).
There was a trend towards improved FFS in the A+I arm versus the I arm in high-risk patient groups; however, the toxicity profile was more favorable with ibrutinib without ASCT.14 OS was prolonged with both ibrutinib-containing regimens, with 4-year OS rates of 81%, 88% and 90% in Arms A, A+I and I, respectively (A vs I, HR: 0.565; p=0.0019; A vs A+I, HR: 0.587; p=0.0036).
These data indicate that high-dose chemotherapy can be avoided in younger MCL patients, with induction and maintenance ibrutinib (and maintenance rituximab) without ASCT as the preferred first-line option.
ENRICH: Rituximab plus ibrutinib in MCL
The median age at MCL diagnosis is 71 years,15 and aggressive treatment approaches are generally unsuitable for most patients.16–18 The current first-line SoC for older MCL patients includes R-CHOP19 or BR.20 Rituximab maintenance following R-CHOP improves both PFS and OS, and real-world data suggest a similar benefit after BR.19,20 In the second line, BTKis such as ibrutinib are the SoC.21 The addition of ibrutinib to first-line BR has been shown to improve PFS compared with BR alone.22
ENRICH is the first phase II/III study that compared rituximab plus ibrutinib versus rituximab plus the investigator’s choice of chemotherapy (either R-CHOP or BR).23 Eligible patients were aged ≥60 years with previously untreated stage II-IV MCL in need of treatment and not fit enough for high dose chemotherapy. A total of 397 patients were randomized to receive either ibrutinib plus rituximab (IR, n=199) or chemotherapy plus rituximab (R-chemo, n=198). Maintenance rituximab was administered in both arms for two years, while patients in the ibrutinib arm continued ibrutinib later on as monotherapy until disease progression or unacceptable toxicity. The primary outcome was PFS.
Overall, rituximab plus ibrutinib demonstrated superior outcomes.23 At a median follow-up of 47.9 months, the median PFS was 65.3 months with IR compared with 42.4 months with R-chemo (HR: 0.69 [95% CI: 0.52–0.90]; p=0.003) (Figure 3A). Subgroup analyses further highlighted the advantage of IR particularly over R-CHOP (HR: 0.37 [95% CI: 0.22–0.62]), with 5-year PFS rates of 52.4% versus 19.2%, respectively (Figure 3B). The difference was less pronounced when IR was compared with BR (HR: 0.91 [95% CI: 0.66–1.25]; 5-year PFS rates, 50.8% vs 47.4%). PFS was numerically longer with IR in patients with TP53 mutations, and a particular benefit of IR was observed in patients with a Ki67 index of <30%. However, PFS tended to be inferior in the IR arm in patients with blastoid disease (consider small number of n=25). A trend toward improved OS was observed with IR compared with chemo (HR: 0.87 [95% CI: 0.64–1.18]), with 5-year OS rates of 57.7% versus 54.5%.
The rates of grade ≥3 AEs were similar across the treatment arms, occurring in 63.1%, 67.8% and 69.2% of patients receiving IR, BR and R-CHOP, respectively.23 However, grade ≥3 cardiac events were more frequent with IR (22.2%) compared with BR (4.9%) and R-CHOP (13.5%). Grade ≥3 atrial fibrillation was reported in 6.1%, 0.7% and 0% of patients, respectively. Patients treated with IR had a significantly lower incidence of neutropenia (9.1%) than those treated with BR (18.9%) or R-CHOP (21.2%). Additionally, the quality of life (QoL) improved more rapidly in the IR arm.
In summary, ENRICH demonstrated improved PFS for ibrutinib plus rituximab versus chemoimmunotherapy plus rituximab in previously untreated MCL.23 This trial is highly relevant for treatment-naïve older patients who may benefit from avoiding chemotherapy.
GHSG HD21: PET-guided BrECADD in older patients with advanced HL
Older patients with advanced-stage classic Hodgkin lymphoma (HL) face a significant unmet need for effective therapy, as they have poorer outcomes and limited treatment options. Escalated-dose bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone (eBEACOPP) regimen is not feasible in this population due to high treatment-related mortality (~15%). Alternative regimens, such as brentuximab vedotin with doxorubicin, vinblastine and dacarbazine (A-AVD) or doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD), show suboptimal efficacy, with 5-year PFS rates of 67% and 62%, respectively.24
The German Hodgkin Study Group (GHSG) HD21 trial had previously demonstrated high efficacy of positron emission tomography-computed tomography (PET-CT)-guided therapy with brentuximab vedotin, etoposide, cyclophosphamide, doxorubicin, dacarbazine and dexamethasone (BrECADD) in HL patients.25 At ASH 2024, results from a prospective, international, multicenter, single-arm add-on cohort of HD21 focusing on older patients with advanced HL were presented.26
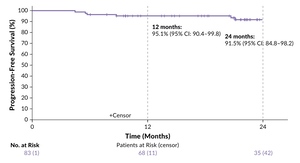
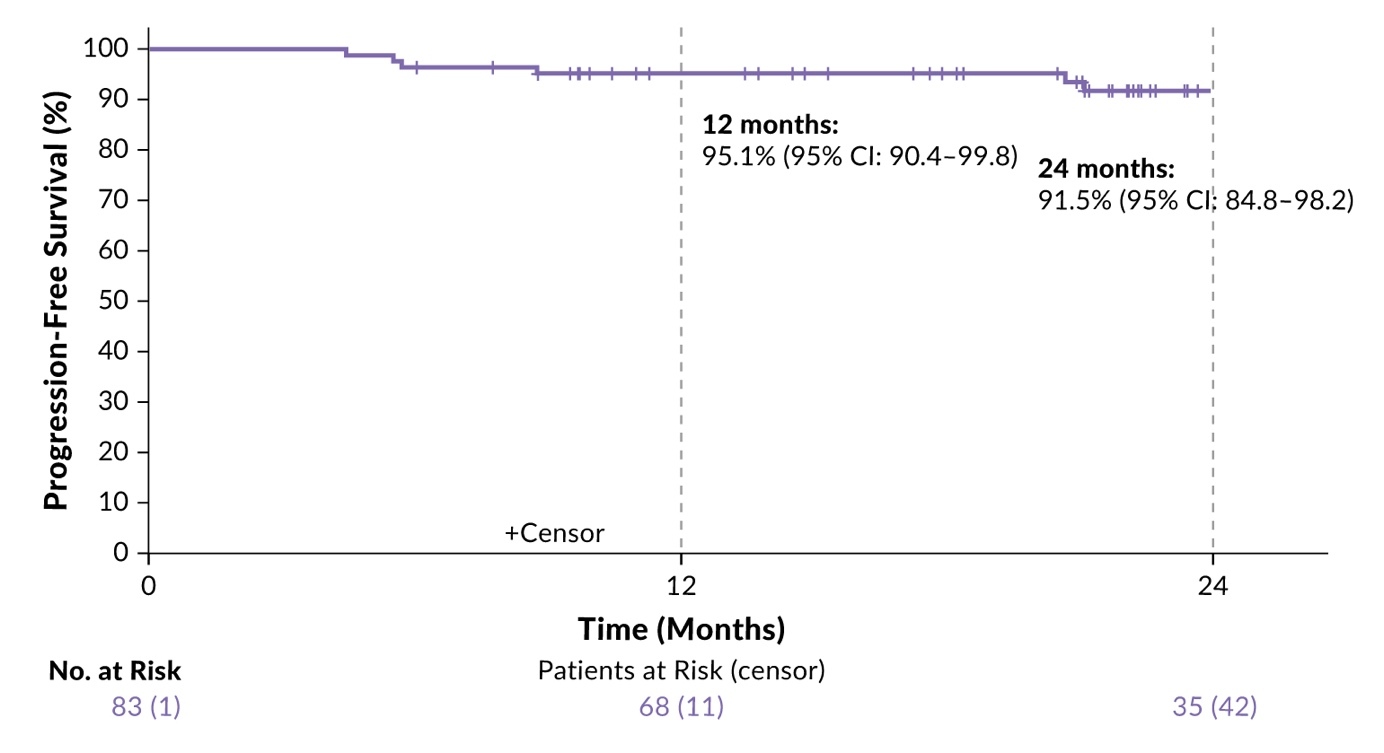
A total of 84 patients (median age 66.5 years, range 61–75 years) was included (ITT population). According to study design, they received two cycles of BrECADD followed by interim PET-CT restaging (PET2).26 Patients with PET2-negative disease received a total of four BrECADD cycles, whereas those with PET2-positive residuals completed six cycles. Consolidation radiotherapy was recommended for PET-positive residuals at EoT. The primary endpoint was the complete response (CR) rate at EoT. The secondary endpoints included AEs, treatment-related morbidity (TRMB), feasibility, PFS, OS and safety.
The study results were promising, with 60% of patients achieving CR after four cycles of therapy based on interim PET-CT, while 22% required six cycles, thus 82% reaching CR at EoT.26 As there were eight patients without centrally evaluated response assessment at EoT, the CR rate in the remaining 76 patients was even higher (87%). Overall, 85% of patients received the scheduled number of cycles - supported by predefined, per-protocol dose reductions - with 12% undergoing consolidating radiotherapy. completed treatment. These high response rates translated into unprecedented PFS outcomes, with a 24-month PFS rate of 91.5% in the ITT population at a median follow-up of 23 months (Figure 4). OS rates at 12 and 24 months were 96.2% and 90.7%, respectively. No treatment-related deaths occurred.
The most common high-grade toxicities were hematologic AEs including anemia (69%) and thrombocytopenia (86%).26 Neutropenic fever occurred in 55% of patients. No grade 5 toxicity was observed. Initially impaired health-related QoL improved during treatment and normalized during the follow-up. These data demonstrate that the BrECADD regimen is feasible for older patients with advanced-stage HL with appropriate dose reductions, addressing an unmet need and offering a new promising SoC in this population, together with the recently published findings from the SWOG S1826-trial assessing the nivolumab plus AVD regimen in this patient population.27
inMIND: Tafasitamab plus rituximab and lenalidomide in relapsed/refractory follicular lymphoma
Finally, important results were reported in the phase III inMIND trial in patients with relapsed and refractory follicular lymphoma (R/R FL).28 Previous studies, such as RELEVANCE, had already shown that rituximab plus lenalidomide is effective as a first-line therapy for FL,29 while L-MIND demonstrated the efficacy of anti-CD19 mAb tafasitamab plus lenalidomide in R/R diffuse large B-cell lymphoma (DLBCL).30 The inMIND study further investigated a combination of rituximab, lenalidomide and tafasitamab versus rituximab plus lenalidomide in patients with FL and marginal zone lymphoma (MZL). At ASH 2024, the primary planned primary analysis of the study was presented.28
This phase III, double-blind, placebo-controlled, international, multicenter, randomized study enrolled patients (aged ≥18 years) with grade 1–3A FL or MZL who had received ≥1 prior line of therapy, including anti-CD20 mAb.28 Patients (n=548) were randomized 1:1 to receive tafasitamab (12 mg/kg; n=273) or placebo (n=275) intravenously (on days 1, 8, 15 and 22 of 28-day cycles 1–3 and days 1 and 15 of cycles 4–12) in combination with the standard dosing of lenalidomide plus rituximab for up to 12 cycles. The primary endpoint was PFS in the FL population. Secondary endpoints included CR rate by PET in fluorodeoxyglucose (FDG)-avid population, OS, PFS, overall response rate (ORR), duration of response, safety, QoL and time to next treatment (TTNT).
The data revealed a clear benefit with respect to the primary endpoint of PFS with the triple therapy.28 At a median follow-up of 14.1 months, the median PFS was 22.4 months with tafasitamab plus lenalidomide and rituximab versus 13.9 months with placebo plus lenalidomide and rituximab (HR: 0.43 [95% CI: 0.32–0.58]; p<0.0001) (Figure 5). Consistent benefits with tafasitamab were observed across all prespecified subgroups, including difficult-to-treat populations such as patients with POD24 or those refractory to prior anti-CD20 mAb.
Furthermore, tafasitamab significantly improved the PET-CR rate (49.4% vs 39.8%; odds ratio [OR]: 1.5 [95% CI: 1.04–2.13]; p=0.0286) and ORR (83.5% vs 72.4%; OR: 2.0 [95% CI: 1.3–3.02]; p=0.0014) versus placebo.28 TTNT was also significantly prolonged with tafasitamab compared with placebo, with a median not reached versus 28.8 months (HR: 0.45 [95% CI: 0.31–0.64]; p<0.0001). The OS data were immature at the time of this analysis, with a trend favoring tafasitamab (HR: 0.59 [95% CI: 0.31–1.13]).
In summary, the first study to evaluate a combination of two mAbs targeting CD19 and CD20 in FL demonstrated a 57% reduction in the risk of progression, relapse or death with tafasitamab plus lenalidomide and rituximab, suggesting that the triple therapy combination could become a new SoC for this patient population.28
Conclusions
At the 66th ASH Annual Meeting and Exposition, several studies with potentially practice-changing results for the treatment of leukemia and lymphoma were presented.
-
Fixed-duration venetoclax plus acalabrutinib significantly improved PFS in treatment-naïve CLL.1,2
-
Ibrutinib-based therapy without ASCT is an effective first-line option for younger patients with MCL.14
-
Ibrutinib plus rituximab was established as a superior frontline regimen for older MCL patients compared to chemoimmunotherapy.23
-
PET-guided BrECADD was validated as a feasible and highly effective regimen for older patients with advanced HL.26
-
The addition of tafasitamab to rituximab and lenalidomide significantly improved PFS in R/R FL.28
Conflict of interest
Adrian Schmidt received honoraria from Takeda, Janssen, Celgene, Sobi, Roche, Sanofi, Amgen, BeiGene, Novartis, Incyte, Eli Lilly and Bayer. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_in_the_amplify_study.jpeg)
_in_patients_receiving_ibrutinib_plus_chemoimmunotherapy_with_(.jpeg)
_versus_rituximab.jpeg)
_in_the_inmind_trial.jpg)
_in_the_amplify_study.jpeg)
_in_patients_receiving_ibrutinib_plus_chemoimmunotherapy_with_(.jpeg)
_versus_rituximab.jpeg)
_in_the_inmind_trial.jpg)