Dear Colleagues,
Recent advancements in the treatment of muscle-invasive bladder cancer (MIBC) have sparked a paradigm shift in perioperative care, as discussed at the Swiss Oncology and Hematology Congress (SOHC) 2024. With neoadjuvant chemotherapy for decades firmly established as the standard care for cisplatin-eligible patients,1–4 significant strides are being made to refine treatment strategies, integrating novel therapies to further optimize patient outcomes.
Neoadjuvant chemotherapy as standard of care
Neoadjuvant chemotherapy offers a clear survival advantage over adjuvant chemotherapy, with studies showing a 5–8% overall survival (OS) improvement over five years for cisplatin-eligible patients.1 This approach, typically implemented through multidisciplinary evaluations and expertise from experienced centers, now serves as a critical component of MIBC treatment planning. Its benefits are multifaceted, targeting micro-metastatic disease when the tumor burden is minimal, improving patient tolerance and delivering enhanced compliance prior to cystectomy. Additionally, neoadjuvant chemotherapy reveals the tumor chemosensitivity, supported by pathological evaluations after surgery. However, potential drawbacks, including delays to cystectomy due to complications, remain a consideration. Despite these concerns, historical evidence clearly demonstrates the superior efficacy of integrated chemotherapy compared with surgery alone — an impact that persists despite evolving treatment protocols.
Insights from the VESPER trial
The data from the VESPER trial led to reshaping of neoadjuvant chemotherapy standards, challenging the once-common cisplatin/gemcitabine regimen with a preference for dose-dense MVAC (methotrexate, vinblastine, doxorubicin and cisplatin).5 These data reveal that dose-dense MVAC provides superior outcomes, including improved progression-free survival (PFS) at three years and OS at five years.5 Although the trial aimed for six cycles, real-world application often limits treatment to four cycles due to toxicity concerns.5 Ensuring completion of these four cycles is critical to maximizing efficacy.5 Dose-dense MVAC also offers benefits in tumor downstaging and pathological response rates, despite higher hematological toxicity.5
The role of pCR
Pathological complete response (pCR) remains a widely used endpoint in clinical trials, but its correlation with OS is under scrutiny.6 Although pCR rates remain below 50%, trials continue to explore its relevance in predicting long-term outcomes.6 This has led to a movement toward integrating advanced systemic treatments, such as immunotherapy, antibody-drug conjugates and novel drug classes, to overcome the limitations of current chemotherapies.6 Guidelines differ on the use of dose-dense MVAC. The National Comprehensive Cancer Network (NCCN)4 includes it as a preferred regimen, whereas the European Society for Medical Oncology (ESMO)1 and European Association of Urology (EAU)2 guidelines have not implemented it as a preferred strategy because they have not yet been updated after the OS data from VESPER. This ongoing debate emphasizes that, while pCR is valuable, it may not be the ultimate measure of treatment efficacy.
Novel approaches and emerging trials
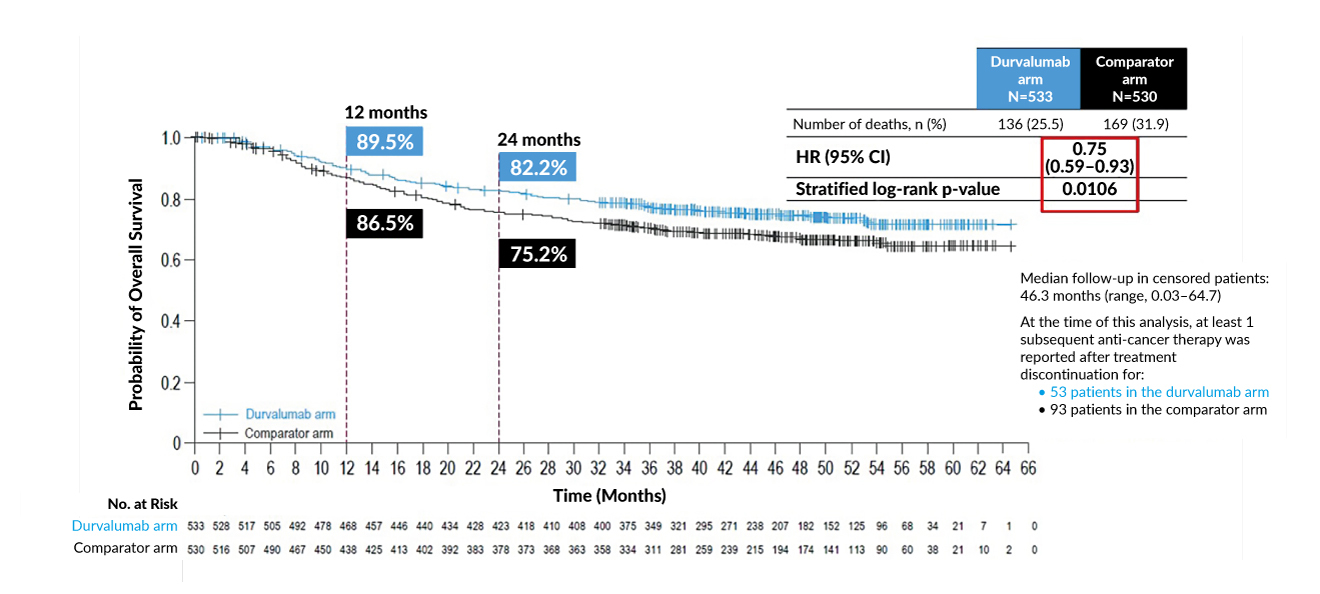
Exciting advancements in perioperative strategies are reflected in recent trials like the NIAGARA study.7 Using a combination of gemcitabine, cisplatin and durvalumab, in both neoadjuvant and adjuvant settings, the trial demonstrated significant benefits in event-free survival and OS (Figure 1).7 These findings solidify the potential of incorporating immunotherapy within treatment protocols, setting a precedent for future guidelines.7 Equally promising are developments in circulating tumor DNA (ctDNA) testing as a tool for minimal residual disease (MRD) detection.8 Ongoing studies like IMVIGOR-011 are pioneering ctDNA-guided treatment pathways, which could fundamentally enhance decision-making for adjuvant therapies.
Addressing challenges for cisplatin-ineligible patients
There is a critical treatment challenge for cisplatin-ineligible patients, a group often characterized by frailty, renal insufficiency or upper tract disease leading to kidney loss. This population frequently experiences poor outcomes, highlighting the urgency of alternative therapies. Modern platinum-free regimens have shown encouraging pCR rates of 30–40%, providing hope for these high-risk cases.9–13
Innovations like TAR-200, an intravesical drug delivery system that provides local continuous gemcitabine release within the bladder, evaluated in the SunRISe-4 study, represent a novel approach for delivering gemcitabine directly into the bladder.14 Combined with anti-PD-L1 therapy, such strategies are breaking new ground, particularly for patients ineligible for conventional treatments.14
Unanswered questions and future directions
In summary, despite significant progress, many questions remain unanswered. Should immunotherapy be prioritized in neoadjuvant, adjuvant or both settings? Do patients who achieve pCR require adjuvant therapy? Most critically, how can we best leverage tools such as ctDNA to personalize treatment strategies and achieve optimal outcomes? Prospective ctDNA-guided trials on the horizon and a growing emphasis on integrating innovative therapies represent the future potential of urogenital cancer treatment. The insights shared at SOHC 2024 highlight the ongoing evolution in this field, driving progress toward more effective, patient-centric care. Looking ahead, these advancements hold the promise of transforming outcomes for patients with MIBC, paving the way for a new era in oncology.
PD Dr Ursula Vogl
Editor-in-Chief
Senior physician oncologist
Oncology Institute of Southern Switzerland and
Ente Ospedaliero Cantonale
Bellinzona, Switzerland
ursula.vogl@eoc.ch
Conflict of interest
The author received advisory board and speaker honoraria from Astellas, Roche, Janssen, Sanofi, Bayer, Merck, MSD, BMS, Pfizer, Novartis AAA and healthbook.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.