Introduction
Bruton tyrosine kinase (BTK) is a key component of multiple signaling pathways downstream of the B-cell antigen receptor (BCR) involved in the development and survival of B cells.1 Highly expressed in a range of immune cells, particularly within the lymphoid lineage, BTK is an effective target for antitumor drugs due to its impact on the tumor microenvironment. In recent years, BTK inhibitors have reshaped the treatment landscape for B-cell malignancies, including chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), Waldenström macroglobulinemia (WM) and marginal zone lymphoma (MZL). While most patients experience prolonged remission with currently available covalent BTK inhibitors, many eventually discontinue treatment because of disease progression or intolerance.2 Pirtobrutinib, a highly selective noncovalent BTK inhibitor, has demonstrated clinical activity in patients with various B-cell malignancies after disease progression on a covalent BTK inhibitor.3,4 This review provides an overview of currently available BTK inhibitors, focusing on recent updates from clinical studies assessing pirtobrutinib in patients with B-cell malignancies.
Mechanisms of resistance to covalent BTK inhibitors
Introducing the first-in-class covalent BTK inhibitor, ibrutinib has led to significantly improved clinical outcomes in patients with various B-cell malignancies, both as monotherapy and in combination with other antitumor regimens.5–16 Despite its effectiveness, up to 60% of long-term treated patients develop resistance due to mutations, particularly the C481S mutation in the ibrutinib binding site of BTK. To address this challenge, a new generation of BTK inhibitors has been developed to overcome resistance toward ibrutinib and reduce its side effects.17 Second-generation covalent inhibitors such as acalabrutinib18–20 and zanubrutinib21–24 offer higher selectivity, resulting in fewer side effects compared with ibrutinib. However, these are also associated with resistance due to resistance mutations, especially C481S, which reduces the binding avidity of the drugs.2 Other approaches to overcome BTK inhibitor failure include combining a BTK inhibitor with other agents that target alternative signaling pathways, such as venetoclax or rituximab.25 Furthermore, third-generation, non-covalent BTK inhibitors, such as pirtobrutinib, nemtabrutinib26 and vecabrutinib,27 are currently being explored to overcome resistance to irreversible BTK inhibitors and improve treatment outcomes in patients with B-cell malignancies. These reversible inhibitors bind noncovalently to BTK a specific pocket in the catalytic domain through weak, reversible hydrogen bonds or hydrophobic interactions, distant from the C481 residue.28,29
Pirtobrutinib: clinical data
The most recently approved BTK inhibitor, pirtobrutinib, is a highly selective, noncovalent BTK inhibitor that blocks both wild-type and C481-mutant BTK with equal low nanomolar potency with a favorable oral pharmacology.30 The clinical efficacy and safety of pirtobrutinib were evaluated in several types of B-cell malignancies (Table 1).
The phase I/II BRUIN study enrolled a total of 778 adult patients with B-cell malignancies; of these, 166 patients had MCL, 317 had CLL/SLL and 295 had other B-cell malignancies including follicular lymphoma (FL) and WM, among others.34 In the phase I dose-escalation portion of the study, patients received pirtobrutinib in 28-day cycles at a dose ranging from 25 mg to 300 mg once daily.45 The phase II part used the recommended phase I dose of 200 mg once daily, until disease progression, unacceptable toxicity or withdrawal.
Mantle cell lymphoma
Based on the findings from the BRUIN study, pirtobrutinib is indicated as monotherapy for the treatment of adult patients with relapsed or refractory (R/R) MCL who have received at least two prior lines of systemic therapy, including an anti-CD20 antibody and a BTK inhibitor, and those ineligible for chimeric antigen receptor (CAR) T-cell therapy.3,30,46 In the primary efficacy cohort of 90 adult patients with MCL previously treated with a BTK inhibitor, pirtobrutinib monotherapy achieved an overall response rate (ORR) of 57.8%, with 57.1% of responders maintaining response at 12 months.3 At a median follow-up of 12 months, the median duration of response (DoR) was 21.6 months among the 52 responders. Pirtobrutinib was also associated with an encouraging 18-month overall survival (OS) rate of 59.3%.
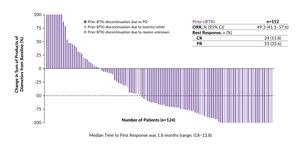
In the latest update of this study, pirtobrutinib demonstrated durable efficacy in the heavily pretreated group of MCL patients.31 The ORR was 49.3%, including a complete response (CR) rate of 15.8% and a partial response (PR) rate of 33.6%, in an expanded cohort of 152 patients (Figure 1). At a median follow-up of 15.9 months and 24.2 months, respectively, the median progression-free survival (PFS) was 5.6 months and the median OS was 23.5 months.
A small subset of patients naïve to a covalent BTK inhibitor (n=14) demonstrated favorable outcomes, with 85.7% of patients having an overall response.31 The median PFS and OS were not evaluable, and the 6-month PFS and OS rates were both 92.3%.
The safety profile of pirtobrutinib was manageable with longer follow-up.31 In the safety cohort (n=166), the most common any-grade adverse events (AEs) were fatigue in approximately one-third of patients (grade ≥3: 3.0%) and diarrhea in 22.3% of patients (no grade ≥3 events). The most frequent any-grade AEs of special interest included infections (42.8%), bruising (16.3%) and rash (14.5%). Discontinuations and dose reductions due to treatment-related AEs (TRAEs) were rare and occurred in 3% and 5% of patients, respectively.
Based on these encouraging results, the benefit of pirtobrutinib is being further assessed in this patient population. The ongoing phase III BRUIN MCL-321 trial aims to investigate whether pirtobrutinib is superior to the investigator’s choice of covalent BTK inhibitor (ibrutinib, acalabrutinib or zanubrutinib) in approximately 500 patients with previously treated but BTK inhibitor-naïve MCL.32 The primary endpoint of the study is PFS per Lugano criteria, assessed by an independent review committee (IRC).
Chronic lymphocytic leukemia
Pirtobrutinib is also approved by the Food and Drug Administration (FDA) for treating patients with CLL who have received at least two prior lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor.47 The BRUIN study included 247 patients with CLL/SLL who had previously received a BTK inhibitor; of these, 100 patients (40%) also received a BCL-2 inhibitor.33 The ORR was 73%, irrespective of the prior exposure to a BCL-2 inhibitor. In the overall efficacy cohort, the median PFS was 19.6 months, with slightly shorter PFS among patients who had previously received both a BTK inhibitor and a BCL2 inhibitor compared with those who had received a BTK inhibitor but not a BCL2 inhibitor (median, 16.8 months vs 19.6 months). At a median follow-up of 22.6 months, the 12-month OS rate was 86.0% and the 18-month OS rate was 80.5% in the overall population.
In an updated analysis at a median follow-up of 30 months, pirtobrutinib continued to show favorable results in patients with R/R CLL/SLL, suggesting that BTK inhibition may be a valuable sequencing approach in this clinical setting.34 Around 80% of patients achieved an overall response, with ORR ranging from 79.7% among patients who previously received a BCL-2 inhibitor (n=128) (Figure 2A) to 83.1% among patients who were naïve to BCL-2 inhibition (n=154) (Figure 2B). This subset of patients also demonstrated improved survival outcomes compared with the BCL-2 inhibitor-exposed group, with a median PFS of 23.0 months versus 15.9 months (24-month rates, 48.3% vs 24.3%) and a median OS of not evaluable in both populations (24-month rates, 83.1% vs 60.6%).
When stratified by prior receipt of a BTK inhibitor, patients with previous exposure to BTK inhibition had a consistent median PFS of 19.4 months at the data cut-off, with 38.6% of patients progression-free at 24 months.34 The median OS in this patient population remained unevaluable and 73.2% of patients were still alive at 24 months. Patients who were naïve to BTK inhibition (n=35) achieved considerably improved survival outcomes, with median PFS and OS not evaluable at a median follow-up of approximately 30 months; the 24-month PFS rate was 81.8% as per investigator and the 24-month OS rate was 88.0%.35 The ORR was high at 94.3.% per investigator assessment, with one patient achieving a CR.
The updated safety analysis showed no new safety concerns after a median time on pirtobrutinib therapy of 18.7 months.34,48,49 The most common treatment-related AEs (TRAEs) were fatigue (36.9%), neutropenia (34.4%), diarrhea (28.4%), cough (27.3%) and contusion (26.2%). Rates of TRAEs of hemorrhage, hypertension and atrial fibrillation or flutter remained low. TRAEs led to dose reductions in 3.9% of patients and discontinuation in 2.5% of patients.
Pirtobrutinib is currently being further investigated in patients with CLL/SLL in various settings. Recently, initial results were reported from a phase II study (NCT05536349) assessing a time-limited, triplet combination of pirtobrutinib, venetoclax and obinutuzumab as first-line treatment for patients with CLL, demonstrating a very high rate of undetectable minimal residual disease (MRD) at six months of therapy.40 In this study, 40 patients received pirtobrutinib continuously for 13 cycles, while obinutuzumab was administered for six cycles and venetoclax standard ramp-up was initiated at cycle 2 to the target dose and continued until end of cycle 13. At the end of cycle 7 (n=39), the rate of undetectable MRD at 10-6 sensitivity was 79% in the peripheral blood and 64% in the bone marrow, with these rates being at 92% and 90% for undetectable MRD at 10-4 sensitivity. By the end of cycle 13 (n=20), 90% of patients achieved undetectable MRD at 10-6 sensitivity in the peripheral blood and 85% in the bone marrow; undetectable MRD at 10-4 sensitivity was observed in 100% and 95% of patients. Notably, no patient has experienced disease progression or died at a median follow-up of nearly 12 months. In terms of safety, the AE profile was similar to prior studies with combination regimens in frontline CLL. Grade 3−4 neutropenia and thrombocytopenia were most frequent and occurred in 60% and 18% of patients, respectively. Dose reduction of pirtobrutinib occurred in 25% of patients and of venetoclax in 23% of patients, most commonly due to neutropenia.
Several ongoing phase III trials are further investigating pirtobrutinib in patients with CLL/SLL. In the frontline setting, the BRUIN CLL-313 trial aimed to assess pirtobrutinib versus bendamustine plus rituximab in approximately 250 untreated patients.36 The BRUIN CLL-314 will compare pirtobrutinib head-to-head with ibrutinib in approximately 650 BTK inhibitor-naïve patients.37 In patients with previously treated CLL/SLL, the BRUIN CLL-321 study will assess pirtobrutinib versus investigator’s choice of idelalisib plus rituximab or bendamustine plus rituximab in around 250 patients with BTK inhibitor-pretreated disease,38 while the BRUIN CLL-322 trial aims to explore fixed duration pirtobrutinib plus venetoclax and rituximab versus venetoclax and rituximab in 600 patients with previously treated CLL/SLL, including those who have received a covalent BTK inhibitor.39
Richter transformation
The phase II BRUIN included a subgroup of 82 patients with Richter transformation, an aggressive form of diffuse large B-cell lymphoma that occurs in up to 10% of patients with CLL.41 At baseline, the median age in this cohort was 67 years, with two-thirds of patients being male. Overall, 90% of patients received at least one previous Richter transformation-directed therapy and 74% had been treated with a covalent BTK inhibitor in a previous line. The results showed an ORR of 50% in this difficult-to-treat population, with a CR rate of 13%. Eight patients with ongoing responses proceeded to stem cell transplantation. The median PFS was 3.7 months and the median OS was 12.5 months, with a 2-year OS rate of 33.5%. These data highlight the promising clinical activity of pirtobrutinib in patients with Richter transformation, most of whom had previously been treated with Richter transformation-directed therapy, including covalent BTK inhibitors.
Antitumor activity of pirtobrutinib in other B-cell malignancies
Treatment options for patients with B-cell malignancies have been limited after a prior covalent BTK inhibitor, including for conditions such as MZL, FL and WM. In the BRUIN study, pirtobrutinib demonstrated promising efficacy and safety in poor-prognosis patients who had previously undergone therapy with anti-CD20 antibodies and covalent BTK inhibitors.30 In detail, among 36 patients with R/R MZL, all of whom were previously treated with an anti-CD20 antibody, 50% achieved an overall response, with one patient having a CR.42 In this population, the median PFS was 16.5 months and the median OS was not reached; at 24 months, 77.5% were still alive. Among the 26 patients who had received a prior covalent BTK inhibitor, the ORR was 46.2%.
Encouraging results were also reported in a cohort of 48 patients with FL, where pirtobrutinib yielded an ORR of 50%, including seven patients achieving a CR.43 With a median follow-up of 18.4 months among the responders (n=24), the median DoR was 5.5 months and the estimated 18-month DoR rate was 41.0%. Data further showed that at 18 months, 32.3% of patients were progression-free and 78.3% remained alive. In the subset of four patients treated with a prior covalent BTK inhibitor, three had PR and one had stable disease.
The BRUIN study enrolled 80 patients with WM, including 63 who had received a prior covalent BTK inhibitor and 17 who were naïve to a covalent BTK inhibitor.44 Following treatment with pirtobrutinib, the major response rate was 71.3% in the overall cohort. In patients with prior exposure to a BTK inhibitor, 66.7% had a major response and 23.8% had a CR plus very good partial response (VGPR). In this subset, the median PFS was 19.4 months and the median OS was not estimable, with 18-month rates of 57.1% and 81.7%, respectively. The BTK inhibitor-naïve patients showed an improved major response rate at 88.2% and a CR plus VGPR rate of 29.4%. Notably, two-thirds of patients who previously received a covalent BTK inhibitor and chemoimmunotherapy (n=50) experienced a major response.
Safety data indicated that pirtobrutinib was generally well-tolerated across the studied groups, with low rates of grade ≥3 AEs and discontinuation due to drug-related toxicity.42–44 The most frequent TEAEs of any grade were diarrhea (20−36%) and fatigue (25−33%). Neutropenia was the most frequent grade ≥3 AE, reported in 15−20% of patients with FL and WM and around 28% of patients with MZL. TEAEs of hemorrhage/hematoma, hypertension and atrial fibrillation/flutter were rare. Overall, rates of discontinuations and dose reductions due to TRAEs were low.
Resistance to pirtobrutinib
Recent studies have identified various non-C481S BTK mutations, as well as mutations in PLCγ2, in patients who experienced disease progression while on pirtobrutinib therapy.50,51 Among these are kinase-dead mutations, such as V416L, C481R, M477I and M437R, which can signal downstream despite reduced BTK autophosphorylation at Y223, a marker of BTK catalytic activity. These mutations are frequently found in patients treated with zanubrutinib or pirtobrutinib but are uncommon in those resistant to ibrutinib.50–52 Gatekeeper mutations at T474 have also been identified in patients who progressed following treatment with acalabrutinib and pirtobrutinib.50,51
Conclusion
Pirtobrutinib has demonstrated significant antitumor activity in R/R B-cell malignancies, with most patients who were refractory to prior covalent BTK inhibitors responding to this treatment.3,30,33 The safety profile of pirtobrutinib is manageable, even for patients intolerant to previous covalent BTK inhibitors, suggesting its potential use after toxicity-related discontinuation. These findings indicate that reinstating BTK inhibition with pirtobrutinib is an effective and safe strategy for patients with MCL who were previously exposed to a covalent BTK inhibitor.
Conflict of interest
Adrian Schmidt received honoraria from Takeda, Janssen, Celgene, Sobi, Roche, Sanofi, Amgen, Beigene, Novartis, Incyte, Eli Lilly and Bayer.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_previousl.jpeg)

_previousl.jpeg)