Introduction
Older age is among the most important risk factors for cancer overall, as well as for many individual types of cancer. Approximately 50% of all cancers occur in the older population, with cancer incidence and mortality rates increasing more than 10-fold in individuals aged ≥65 years compared with the younger age group globally.1,2 In Switzerland, cancer-related mortality was >5 times higher in older patients compared with the younger population in 2022.3 As the world’s population is aging rapidly, the socioeconomic burden of cancer has become an increasingly relevant health burden. The treatment of older patients with cancer presents a multifaceted challenge.4–6 Frailty, often exacerbated by age-related physiological decline and the presence of comorbidities, complicates treatment decisions by impacting tolerance to therapeutic interventions and overall prognosis. Older people are often underrepresented in clinical trials, which creates another limitation in treatment options due to the lack of evidence-based data and guidelines in this population, especially in later lines of therapy. Furthermore, older age is associated with significant differences in tumor pathobiology, including molecular and genetic profiles, histopathological presentation and age-related changes in the tumor microenvironment, which may have an impact on disease progression, response to therapy and clinical outcome.7 Together, this indicates an urgent need for predictive models that could provide diagnostic and prognostic information relevant to older patients with cancer.
Tumor-derived organoids in drug screening
Two-dimensional (2D) cultures of primary patient-derived tumor cells and cancer cell lines have been extensively used for studies on oncogenesis mechanisms and drug screening. While remaining a valuable tool in preclinical research, 2D models are unable to correctly mimic the cellular composition of tumor tissue, epithelial polarity and the complex extracellular microenvironment and therefore have limited predictive capacity for processes in the living organism. Organoid technology has emerged as a promising frontier in cancer treatment, offering a versatile platform for personalized medicine and drug development.6,8–11 By recapitulating three-dimensional (3D) tissue architecture, cell-matrix interactions and tumor heterogeneity, organoids have been shown to provide an accurate representation of individual tumors and preserve tumor characteristics, such as phenotype and mutational signatures, thus enabling precise modeling of tumor behavior. Ex vivo screening of organoids derived from patient tumor tissue allows the prediction of individual tumor responses to therapeutic agents, including chemotherapy, immunotherapy and targeted therapy. Treatment efficacy can be evaluated based on various endpoints, such as organoid size, cell viability, cytokine production and omic-based analysis, inter alia. Importantly, and in contrast to many other ex vivo and in vivo models, organoid technology allows for automated high-throughput and high-content screening,12 making it a very efficient tool for the identification of individual therapeutic strategies tailored to specific tumor profiles with the best chance of success, simultaneously excluding ineffective treatments. Moreover, organoids can serve as tools for studying tumor biology, unraveling the mechanisms of drug resistance and advancing our understanding of cancer progression.
Many studies have demonstrated high predictive value and a statistically significant correlation between patient’s clinical response and tumor organoid response.11,13,14 A pooled analysis of 17 publications showed that patient-derived organoids could predict clinical response in patients with different types of cancer with a sensitivity and specificity of 0.81 (95% CI: 0.69–0.89) and 0.74 (95% CI: 0.64–0.82), respectively.15 In patients with metastatic gastrointestinal cancers, a highly positive correlation between ex vivo tumor organoid response and patient response have been observed, with 88% positive predictive value and 100% negative predictive value of the organoid screening results.14 A successful guidance of personalized treatment strategy using patient-derived tumor organoids have been reported in a patient with “cancer of unknown primary” with axillary lymph node metastases later classified as triple-negative breast cancer.16 The success rates of organoid culture vary across tumor types and are significantly influenced by the availability of the starting material, tumor cellularity and other factors, justifying further studies on optimization and validation of this approach to expand its clinical translation in personalized medicine.
Molecular mechanisms of aging and importance of experimental models in aging research
Aging is characterized by a plethora of changes at the cellular and tissue levels.17 One of the primary differences between young and aging cells lies in genomic instability.18 As cells age, they accumulate DNA damage due to various factors, such as oxidative stress, environmental exposures, and replication errors. Although cells possess mechanisms to repair DNA damage, these systems become less efficient with age, leading to an increase in the number of mutations and chromosomal aberrations. This genomic instability can trigger apoptosis (programmed cell death) or senescence (a state of permanent growth arrest). Senescent cells, while no longer dividing, secrete pro-inflammatory cytokines and other factors that disrupt tissue homeostasis and promote aging-related pathologies. Epigenetic changes also play a significant role in aging. These include alterations in DNA methylation patterns such as global hypomethylation and regional hypermethylation, and changes in histone modifications. These epigenetic alterations can lead to dysregulated gene expression, which contributes to the functional decline observed in aging cells. Furthermore, aging cells exhibit significant changes in protein homeostasis or proteostasis.19 Proteins are susceptible to various forms of damage, including glycation, oxidation, and misfolding. Non-enzymatic glycation, particularly affecting extracellular matrix (ECM) proteins such as collagen and elastin, leads to increased stiffness and loss of elasticity in tissues, contributing to conditions such as fibrosis and cardiovascular disease. Oxidative stress leads to carbonylation, fragmentation and cross-linking of proteins, which impairs protein function and results in the accumulation of damaged proteins. Mitochondrial dysfunction is a hallmark of aging cells, characterized by a decline in mitochondrial efficiency and an increase in the production of reactive oxygen species (ROS).20 Mutations accumulating in mitochondrial DNA impair the function of the electron transport chain and reduce ATP production. This energy deficit hampers cellular functions and contributes to a gradual decline in tissue and organ performance. Furthermore, increased ROS levels cause oxidative damage to cellular components, including lipids, proteins and DNA, further exacerbating cellular aging and promoting inflammation. At the tissue level, this leads to increased susceptibility to cancer and other diseases, as well as to a decline in stem cell function, which impairs the tissue’s ability to repair and regenerate. Additionally, the aging immune system shifts towards a pro-inflammatory state, known as “inflammaging” that is characterized by chronic, low-grade inflammation at the organism level, contributes to systemic tissue damage and age-related diseases such as cardiovascular disease and Alzheimer’s.21 At the cellular level, inflammaging results from the accumulation of senescent cells that secrete pro-inflammatory cytokines, disrupting local tissue homeostasis and impairing regenerative processes.
Importantly, these age-related changes also affect cell sensitivity to drugs and responses to therapy, necessitating the development of age-specific therapeutic strategies. Experimental models that preserve age-related cell properties are crucial for studying the intricate processes of aging, identifying age-specific molecular signatures and developing therapeutic approaches aimed specifically at older individuals.22 By using primary cell cultures and organoids derived from tissues of younger and older donors, researchers can compare the intrinsic properties of cells from different age groups in a controlled environment, correctly mimicking cellular processes in the adult organism for the purpose of drug screening. High-throughput screening of drugs and interventions using these models can facilitate the development of therapies specifically targeted at older patients, including those with cancer.
Organoid-based drug screening in older cancer patients: A Swiss registry study
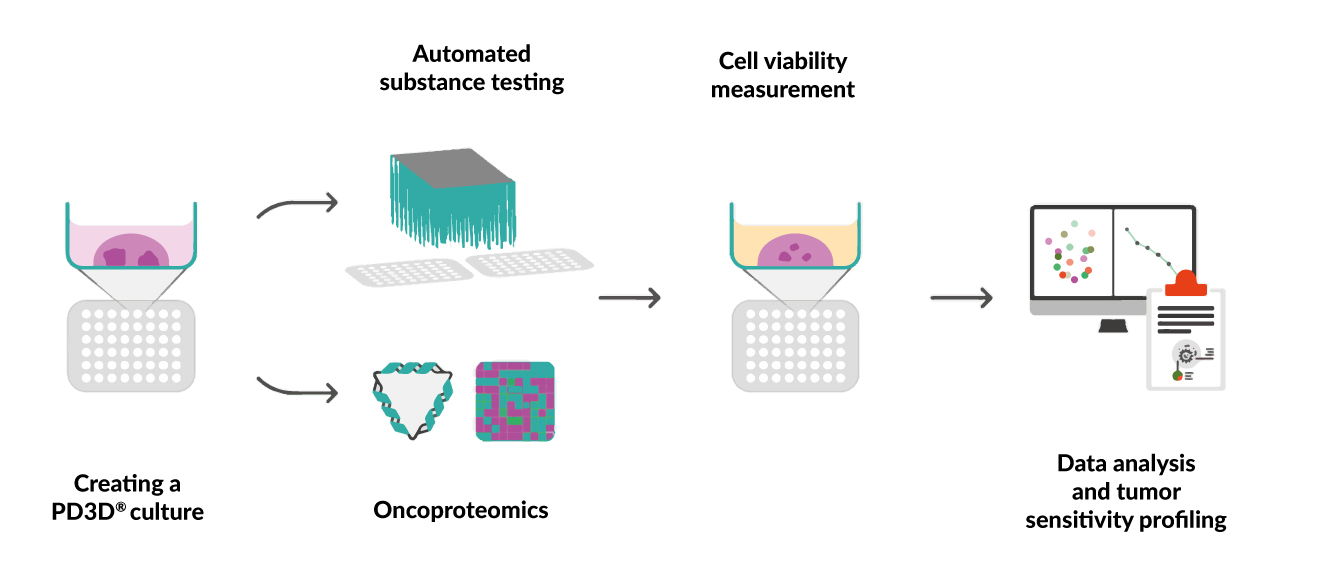
The first ongoing Swiss registry study (SIOG2022-0052) conducted at the Cantonal Hospital Baselland, Liestal, Switzerland, aims to apply organoid technology in the area of geriatric oncology to identify optimal anticancer treatments in older cancer patients in the second or later therapy line setting for whom no standard treatments are available.23 The study enrolls patients aged >65 years with a confirmed diagnosis of advanced cancer. Patient characteristics, including age, body mass index, food intake, weight loss, mobility, neuropsychological problems, polypharmacy and self-perceived health status, are determined at baseline using the geriatric G8 score.24 Tumor tissue biopsy samples freshly collected from advanced localized tumors and metastasis sites are subjected to personalized chemosensitivity assessment using the patient-derived 3D (PD3D®) cell culture model (CELLphenomics GmbH). Following mechanochemical preparation of the tissue, automated testing of standard-of-care and experimental drugs and cell viability measurements are performed in a 384-well format using the Reverse Clinical Engineering® procedure (ASC Oncology), which allows individual sample processing on average in 28 days (Figure 1).25 In parallel, tissue samples are also embedded for pathology and subjected to oncoproteomic analysis. The obtained data are evaluated using specialized software and an efficacy profile is created for each individual tumor for each tested drug or combination therapy. To support future preclinical and translational research, both fixed tissue and viable PD3D® models are stored in a biobank. An evaluation of the data from this study is in progress.
Conclusions
The integration of organoid technology into cancer treatment holds immense potential for the implementation of personalized approaches in clinical practice. The advantages of this method include the evaluation of drug sensitivity under conditions recapitulating tumor tissue architecture and heterogeneity, as well as high-throughput screening in a therapy-relevant timeframe. In older patients, organoid-based drug screening can help identify the optimal treatment strategy, reduce unnecessary treatment-related toxicity, improve patient outcomes and develop individually tailored treatment recommendations for this patient population, which is underrepresented in clinical trials. This ongoing academic registry study is hypothesis-generating and potential results require validation through controlled trials.
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki and was approved by the local Ethics Committee.
Consent for publication
General written consent was obtained from the patients for publication of this study.
Conflict of interest
PD Dr Marcus Vetter received honoraria for consultancy from GSK, Roche, Novartis, ExactSciences, Pfizer, Stemline, AbbVie and ASC Oncology. All other authors have declared that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
All authors have declared that no financial support was received from any organization for the submitted article.
Author contributions
All authors contributed to and approved the final manuscript.