Introduction
Biliary tract cancer (BTC) is a broad term that encompasses a group of malignancies with anatomical and molecular diversity originating from the biliary epithelium. The four anatomical categories of BTC include intrahepatic cholangiocarcinoma (ICC), perihilar cholangiocarcinoma (PCC), distal extrahepatic cholangiocarcinoma (ECC) and gallbladder carcinoma (GBC), which exhibit similar histopathological morphology.1,2 However, the distribution of distinct molecular subtypes differs notably among the four BTC classifications (Figure 1).2 Although BTCs are relatively uncommon, recent epidemiological studies show an increasing worldwide incidence.3,4 BTC incidence rates differ markedly by geographic region, with Asia-Pacific and South American countries showing higher prevalence (ranging from 1.12 cases/100,000 person-years in Vietnam to 12.42 cases per 100,000 person-years in Chile) compared to European and North American countries (ranging from 2.00 to 3.59 cases per 100,000 person-years in Europe and 2.33 to 2.35 cases per 100,000 person-years in North America).4 Early-stage BTCs often remain asymptomatic, leading to diagnoses at advanced stages when curative treatments like tumor resection or liver transplantation are generally not an option. This results in a five-year survival rate of less than 20% for the entire BTC population.3,4 Prognosis is especially poor for patients presenting with distant metastatic spread, which is associated with a five-year overall survival rate of less than 5%.3,5
TCs exhibit significant pathophysiological and molecular heterogeneity, complicating treatment efforts, particularly in advanced cases where surgery is not viable.6 Over 65% of BTC patients present with unresectable disease, and more than half of those who undergo potentially curative surgery experience an incurable tumor relapse.7–10 For many years, the systemic treatment landscape for advanced BTC was largely limited to chemotherapy, with a significant but moderate improvement in overall survival (OS) for patients.1 More recently, molecularly targeted agents for distinct molecular subgroups of BTC have been approved for later-line systemic treatment, the most prominent being FGFR inhibitors for BTCs harboring FGFR2 genomic rearrangements and gene fusions, ivosidenib for BTCs harboring IDH1 R132 hot spot mutations, dabrafenib plus trametinib for BRAF V600E-mutated BTCs, and the anti-programmed cell death protein 1 (PD-1) checkpoint inhibitor pembrolizumab for mismatch repair-deficient/high microsatellite instability (dMMR/MSI-H) BTCs.11 Another emerging molecular target in BTCs is HER2-positivity, with a number of HER2-targeting strategies currently under clinical development.2 The US Food and Drug Administration (FDA) approved the antibody-drug conjugate fam-trastuzumab deruxtecan-nxki for the treatment of adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors, also including BTC, who have received prior systemic treatment and have no satisfactory alternative treatment option.12
For systemic first-line treatment, however, the introduction of chemoimmunotherapy has changed practice for cancer patients with unresectable BTC and is now integral in guideline-recommended treatment regimens.13,14 A significant milestone was achieved with the approval of durvalumab plus chemotherapy with cisplatin/gemcitabine (CG) for previously untreated patients with advanced and metastatic BTC. The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) granted approval for this combination in 2022, followed by Swissmedic in 2023, based on the pivotal results of the TOPAZ-1 trial.14–17 Subsequently the FDA18 and EMA19 in 2023 and Swissmedic in 202420 approved pembrolizumab in combination with CG in the first-line advanced BTC setting based on results from the phase III KEYNOTE-966 trial (NCT04003636).21 Notably, both TOPAZ-1 and KEYNOTE-966 phase III trials demonstrated a significant and clinically relevant improvement in OS for the triple chemoimmunotherapy combination compared to chemotherapy with CG alone.14,21 Remarkably, the addition of durvalumab to standard chemotherapy regimens represented the first major change in the standard of care for BTC in over a decade.6,14,22 The latest follow-up data of TOPAZ-1 confirm that durvalumab plus chemotherapy doubled the OS rate for patients with advanced BTC in the TOPAZ-1 trial (3-year OS rate was 14.6% [95% CI: 11.0–18.6] in the triple combination arm vs 6.9% [95% CI: 4.5–10.0] in the placebo arm).23,24 These 3-year follow-up results, which were recently presented at the 2024 Annual Cholangiocarcinoma Foundation (CCF) Conference in April, and as an oral presentation by Dr Oh at the European Society for Medical Oncology Gastrointestinal Cancers (ESMO GI) 2024 Congress in June, represent the longest survival follow-up reported for immunotherapy treatment in this setting.23,24
This mini-review presents the latest findings from the TOPAZ-1 trial and provides insights into durvalumab’s mechanism of action. It also explores both clinical trial results and real-world outcomes with durvalumab in combination with CG for patients with advanced BTC.
Antitumor activity of durvalumab
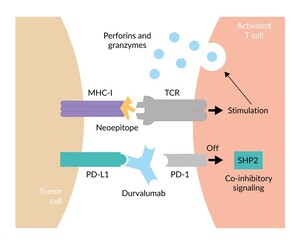
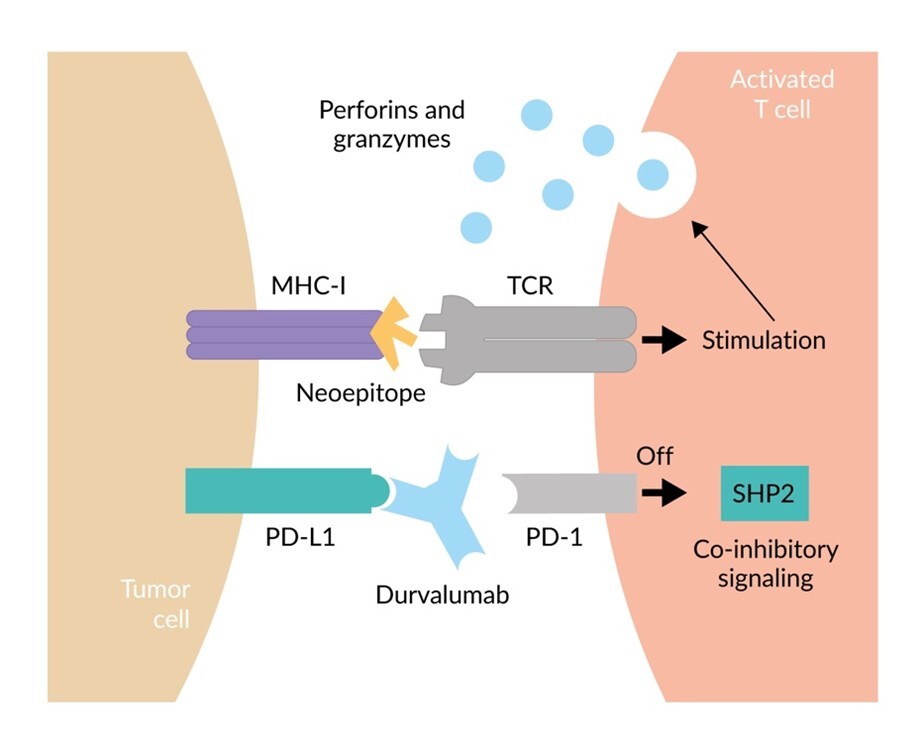
PD-1 is an immunosuppressive receptor on the surface of T cells and other immune cells, playing a critical role in suppressing immune responses and promoting self-tolerance. In the context of cancer, programmed cell death ligand 1 (PD-L1) is frequently overexpressed on malignant tumor cells, driving immune evasion of tumors. Targeting the PD-1/PD-L1 interaction for cancer therapy has been the most important breakthrough for cancer therapy of the last decade, evidenced by multiple approvals of several anti-PD-1 and anti-PD-L1 monoclonal antibodies for cancer treatment in multiple indications, drug combinations and treatment lines. Durvalumab is a human immunoglobulin G1 kappa anti-PD-L1 monoclonal antibody that inhibits the immune-suppressing interaction between PD-L1 and PD-1, thereby preventing the Src homology 2 domain-containing protein tyrosine phosphatase (SHP2)-mediated co-inhibitory signal.25 This allows the neoepitope presented by the major histocompatibility complex (MHC) to amplify a T-cell anticancer immune response, resulting in the release of perforins and granzymes, leading to the destruction of the tumor cell (Figure 2).25,26 Additionally, it may mark tumor cells for destruction or removal through antibody-dependent cellular cytotoxicity or phagocytosis.27
Immunotherapy in combination with chemotherapy in BTC
Research indicates that chemotherapy might work synergistically with anti-PD-L1 antibodies such as durvalumab through various immunomodulatory mechanisms, providing a strong rationale for exploring chemoimmunotherapy combinations in BTC.28 A phase II open-label, single-arm clinical trial evaluated durvalumab in combination with CG in patients with unresectable or metastatic BTC, reporting a median OS of 18.1 months (Table 1).29 Following these findings, the randomized phase III TOPAZ-1 study was conducted.
Durvalumab plus chemotherapy versus placebo plus chemotherapy enhances survival and response rates in advanced BTC
The international, double-blind, placebo-controlled TOPAZ-1 trial randomized patients with unresectable or metastatic BTC to receive either chemoimmunotherapy with durvalumab plus CG or CG plus placebo as first-line treatment in patients with unresectable and metastatic BTC (Table 1).14 The study met its primary endpoint, showing a statistically significant improvement in OS for the durvalumab plus chemotherapy combination (median follow-up: 23.4 months [95% CI: 20.6–25.2]) versus placebo plus chemotherapy (median follow-up: 22.4 months [95% CI: 21.4–23.8]), with a median OS 12.9 versus 11.3 months, respectively (hazard ratio [HR]: 0.76 [95% CI: 0.64–0.91]; p=0.021; Figure 3).30 This OS data represents the first update following an additional 6.5 months of follow-up beyond the initial report.14,30 Additionally, adding durvalumab to CG extended progression-free survival (PFS) (median 7.2 vs 5.7 months; HR: 0.75 [95% CI: 0.63–0.89]; p=0.001; Figure 4) and increased the objective response rate (ORR) (27% vs 19%).14 Rates of grade 3/4 adverse events did not rise with durvalumab plus chemotherapy (75.7% vs 77.8% in the control group), and 12.7% of patients experienced any grade immune-related events compared to 4.7% in the placebo plus chemotherapy arm.14
Durvalumab plus chemotherapy combination improves OS without compromising QoL in advanced BTC patients
A secondary objective of the TOPAZ-1 trial was to evaluate patient-reported outcomes (PROs) between the two treatment groups using the European Organisation for Research and Treatment of Cancer 30-item Quality of Life questionnaire (EORTC QLQ-C30) and a 21-item biliary tract cancer-specific module (EORTC QLQ-BIL21).31 The primary measure of PROs was time to deterioration (TTD), defined as the period from randomization to the first clinically meaningful worsening of a PRO, confirmed at a subsequent visit. High baseline completion rates (>81%) for both questionnaires were sustained (>70% over 28 cycles) across both treatment arms. Initial scores were comparable between groups.31 Analysis revealed no significant decline in quality of life (QoL) for any functional domains or symptom scores with durvalumab plus chemotherapy compared to placebo plus chemotherapy.31 In fact, adjusted mean changes from baseline suggested improved QoL with durvalumab plus chemotherapy. Median TTD for Global Health Status/QoL was slightly longer with durvalumab plus chemotherapy (7.4 months) compared to placebo plus chemotherapy (6.7 months), although not statistically significant (Figure 5).31 Ultimately, the authors of this PRO analysis concluded that adding durvalumab to CG improves OS without compromising QoL, recommending this combination as the new first-line treatment for advanced BTCs.31
Deterioration was defined as an absolute decrease of at least 10 points in global health status or QoL scores on the EORTC QLQ-C30. Participants with disease control (defined as either a complete response, partial response, or stable disease) and with progressive disease were censored at their last patient-reported outcomes assessment, for which data were available. The total number of participants with a time to deterioration event equals the number at risk at baseline minus the number censored at month 24. All participants in both groups received CG in addition to their randomly assigned treatment. The dotted line represents the separation point for the post-hoc piecewise HR analysis.
Real-world findings in advanced BTC patients treated with durvalumab plus chemotherapy
The positive survival impact of first-line durvalumab in combination with CG compared with CG alone has been confirmed in a real-world cohort of patients with locally advanced or metastatic BTC.32 These findings build upon clinical trial data from the TOPAZ-1 trial and provide additional insights into the treatment’s performance in broader patient populations.32 The study included 563 patients with unresectable, locally advanced or metastatic BTC. Of these, 213 were treated with CG alone, while 350 received the combination of CG plus durvalumab. Patients receiving durvalumab in combination with chemotherapy had a median OS of 14.8 months, compared to 11.2 months for those on chemotherapy alone (HR: 0.63 [95% CI: 0.50–0.80]; p=0.0002). The median PFS was 8.3 months for the combination therapy group versus 6.0 months for the chemotherapy-only group (HR: 0.57 [95% CI: 0.47–0.70]; p<0.0001). Multivariate analysis confirmed that the addition of durvalumab to standard chemotherapy is an independent prognostic factor for both OS and PFS, offering significant survival benefits. Notably, patients over 70 years old and those with locally advanced disease experienced the highest survival improvements.32 An exploratory analysis identified neutrophil-lymphocyte ratio (NLR) and disease stage as independent prognostic factors within the durvalumab cohort, with NLR ≤3, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0, and locally advanced disease serving as positive predictive factors for OS.32 In line with the findings of the TOPAZ-1 trial, this real-world study confirms that adding durvalumab plus CG significantly enhances survival outcomes for patients with advanced BTC.32
Durvalumab plus chemotherapy doubles 3-year survival in advanced BTC: Long-term findings from TOPAZ-1
Updated results from TOPAZ-1 reveal that durvalumab plus chemotherapy significantly improves long-term OS for patients with advanced BTC. These data, presented at both the 2024 Annual CCF Conference and the ESMO GI 2024 Congress, represent the longest survival follow-up ever reported for immunotherapy in BTC patients. After a median follow-up of 41.3 months, durvalumab plus chemotherapy reduced the risk of death by 26% compared to chemotherapy alone (HR: 0.74 [95% CI: 0.63–0.87]) (Figure 6)23,24; this was a numerical improvement on the primary analysis (0.80 [95% CI: 0.66–0.97]).14 The median OS was 12.9 months for the immunotherapy plus chemotherapy combination group versus 11.3 months for the chemotherapy-only group.23,24 Notably, the 3-year survival rate showed that 14.6% of patients on the durvalumab-based regimen were alive at three years, compared to 6.9% for chemotherapy alone.23,24 These newest findings from the TOPAZ-1 trial indicate that the number of patients with advanced BTC surviving at the three-year mark was double for those treated with durvalumab and chemotherapy compared to historical data (Figure 7).23,24 Moreover, in participants achieving disease control, the 3-year OS for the durvalumab plus chemotherapy arm was more than double that of the placebo plus chemotherapy arm (17.0% vs 7.6%, respectively), and median OS was improved compared to the full analysis set.24 Subsequent anticancer therapy use, including subsequent immunotherapy, was less frequent in patients in the durvalumab plus chemotherapy arm compared with the placebo plus chemotherapy arm (58.6% vs 83.3%, respectively).24 This significant improvement highlights the poor prognosis typically associated with this disease. For the safety analysis, serious adverse events that were considered to be related to treatment (as assessed by the study investigator) occurred in 15.4% of patients who received durvalumab plus chemotherapy and in 17.3% of patients who received placebo plus chemotherapy (8.6% vs 10.0% in the extended long-term survival group [participants still alive ≥30 months after randomization], respectively).23,24 Overall, the safety profile of the durvalumab combination was consistent with previous analyses, with no new signals identified.23,24 These outcomes underscore the sustained effectiveness of the durvalumab plus chemotherapy combination, solidifying its role as a standard treatment option for this challenging condition.23,24
Current standard of care in advanced BTC
Molecular analysis should be conducted before or during first-line therapy to assess potential options for subsequent treatments as early as possible in advanced disease.1,13
First-line treatment options
For advanced or metastatic BTC, durvalumab in combination with CG has also been newly incorporated as a standard of care for patients with locally advanced or metastatic BTC based on recent clinical trial data from TOPAZ-1, demonstrating improved outcomes.1,13
Second and subsequent lines
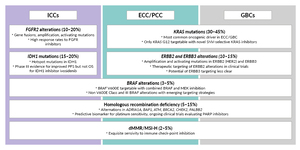
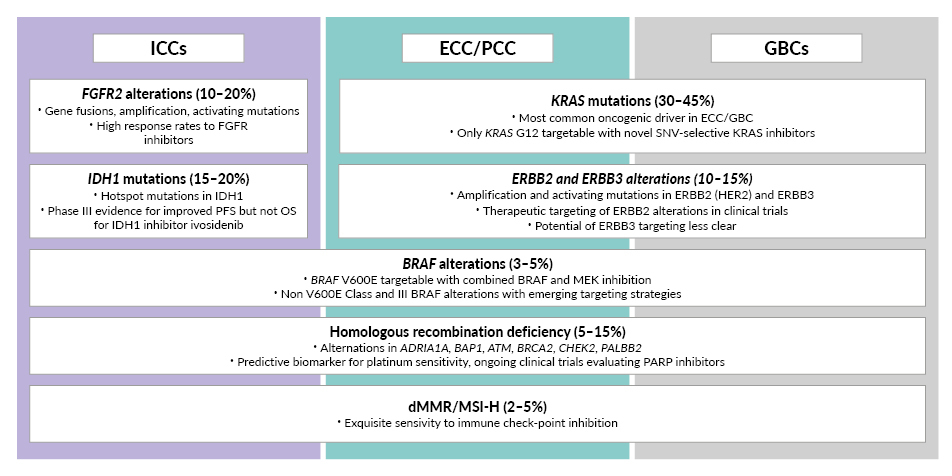
BTC is characterized by a high rate of potentially actionable genomic alterations, with mutational analyses revealing actionable alterations in up to 50% of BTCs.33 Actionable alterations are particularly common in intrahepatic cholangiocarcinomas (ICCs) and include hot spot mutations in isocitrate dehydrogenase 1 (IDH1), genomic rearrangements and fusions involving fibroblast growth factor receptor 2 (FGFR2), and the BRAF V600E mutation. Across all anatomical subtypes of BTC, amplifications and activating mutations in human epidermal growth factor receptor (HER2), as well as targetable and currently non-targetable mutations in Kirsten rat sarcoma viral oncogene homolog (KRAS), are prevalent. Finally, a small subset of BTCs show dMMR/MSI-H, rendering those tumors especially sensitive to immunotherapy with anti-PD-1 antibodies.2,11,33–35 As a result, modern second-line treatment options in BTCs are personalized based on the detection of actionable genomic alterations.1,13 For patients with tumors without actionable molecular alterations, the standard second-line treatment is combination chemotherapy with FOLFOX, based on the randomized phase III ABC-06 trial.1,13,36 Pembrolizumab monotherapy is approved by Swissmedic for tumors with MSI-H or dMMR in adult patients with unresectable or metastatic colorectal cancer (CRC) after prior fluoropyrimidine-based therapy in combination with irinotecan or oxaliplatin and adult patients with metastatic endometrial carcinoma, gastric carcinoma, small bowel carcinoma or cholangiocarcinoma who are progressive after standard therapy and for whom no satisfactory treatment alternatives are available.20 This approval, based on the KEYNOTE-158 trial (NCT02628067), is for cases where the solid tumors have progressed following prior treatment and no satisfactory alternative treatment options exist.13,37,38 Recent results from the phase IIb Nifty trial (NCT03524508) suggest that adding liposomal irinotecan (nal-IRI) to fluorouracil and leucovorin (FL) could be considered a further-line therapy for patients with advanced BTC.13,39
Conclusion and future perspectives
Treatment of advanced and metastatic BTCs remains a challenge. However, the introduction of chemoimmunotherapy with durvalumab plus CG in first-line systemic treatment, together with ground-breaking progress in identifying and targeting actionable molecular alterations in second-line BTC treatment, has greatly enhanced treatment options for patients. The phase III TOPAZ-1 trial has demonstrated a clinically meaningful survival benefit of chemoimmunotherapy, as compared to chemotherapy alone, for patients with advanced BTC. These trial findings, along with supportive real-world data, indicate that durvalumab, in combination with CG, significantly enhances patient survival without compromising patient QoL. The addition of durvalumab has not only become an integral part of the first-line treatment for advanced BTC but also offered renewed optimism for better management and outcomes in this difficult-to-treat patient population.
Looking forward, the sustained effectiveness observed in long-term follow-ups from the TOPAZ-1 trial underscores the potential of cancer immunotherapy to achieve meaningful survival benefits. Furthermore, the importance of molecular analysis in guiding personalized treatment decisions cannot be overstated. As genomic profiling becomes more integrated into clinical practice, targeted therapies tailored to specific mutations will likely play a critical role in second-line treatments. Future research should focus on refining these therapeutic strategies, exploring synergistic combinations and expanding our understanding of BTC’s molecular underpinnings. These efforts will be crucial in improving survival rates and QoL for BTC patients, ultimately transforming the outlook for patients with this aggressive disease.
Conflict of interest
PD Dr Ralph Fritsch has served as an advisor for Bristol-Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pierre Fabre, and has received speaker’s fees from BMS, Servier and Pierre Fabre.
Funding
Preparation of this article was supported by AstraZeneca. The supporting company did not have any decision-making role in the development of the manuscript and did not influence its content in any way.
Author contributions
The author has created and approved the final manuscript.

.jpeg)
_by_treatment_group_.jpg)
_curve_at_3_years.jpeg)
_patients_who_received_durvalumab_and.jpeg)

.jpeg)
_by_treatment_group_.jpg)
_curve_at_3_years.jpeg)
_patients_who_received_durvalumab_and.jpeg)