Dear Readers,
Cancer treatment is a critical area in modern medicine, presenting unique challenges for various patient demographics. One of the most significant hurdles is addressing the specific needs of older patients, who often deal with frailty and multiple comorbidities. These factors complicate therapeutic decisions and exacerbate issues related to treatment tolerance, making it a particularly complex problem to manage effectively. Furthermore, the underrepresentation of older patients in clinical trials leads to a scarcity of evidence-based data, especially for advanced therapy lines. Amidst these challenges, organoid technology emerges as a beacon of hope.1 By accurately replicating the three-dimensional architecture and heterogeneity of tumors, organoids offer a groundbreaking platform for personalized medicine and drug development.1 This third editorial for healthbook TIMES Oncology Hematology 2024 delves into the transformative potential of organoid technology in oncology, with a spotlight on the ongoing Swiss registry study by Häuptle et al., which I co-authored, focusing on optimizing treatments for older cancer patients through personalized organoid-based approaches.
What is organoid technology?
Organoids are three-dimensional constructs that mimic the architecture and function of real tissues and organs. They are derived from stem cells and can be developed from embryonic stem cells, induced pluripotent stem cells, somatic stem cells and cancer cells in specific 3D culture systems. These constructs serve as near-physiological models for studying human diseases, including cancer, and have applications in drug testing and personalized therapy.2 In personalized medicine, organoids derived from patient-specific cells enable the modeling of individual tumor characteristics, facilitating tailored treatment strategies.3 This technology plays a critical role in drug development and testing by providing a high-throughput system for screening potential therapeutics, thus accelerating the discovery of effective drugs while minimizing unnecessary toxicity. Additionally, organoids are invaluable in disease modeling, allowing researchers to study the intricate mechanisms of diseases such as cancer in a controlled environment that closely mimics human tissue architecture.4 Beyond oncology, organoid technology is also being explored for regenerative medicine, toxicology testing and studying infectious diseases, thereby significantly advancing medical research and improving patient outcomes across various fields.
How is organoid technology advancing personalized medicine in oncology?
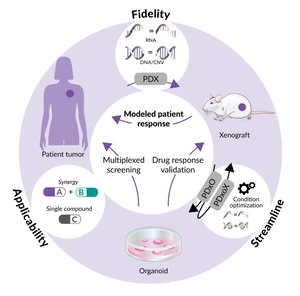
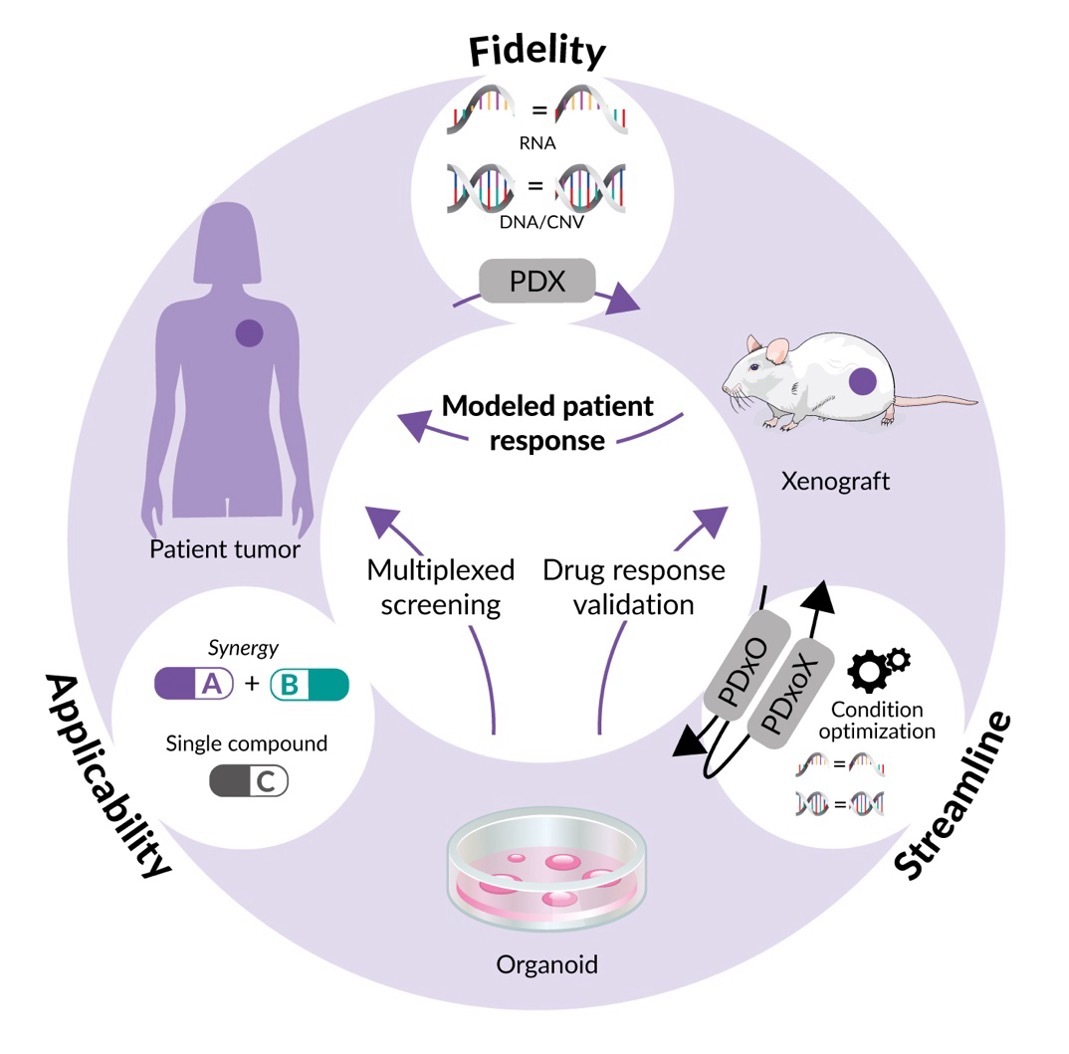
For example, by addressing the complexity of human tumors, such as breast cancer, organoid technology is advancing personalized medicine through the development of patient-derived xenografts (PDXs) and matched organoid cultures (Figure 1).5 These models, including endocrine-resistant, treatment-refractory and metastatic breast cancers, enable long-term growth and maintain high fidelity to the original tumors. By facilitating high-throughput, cost-effective drug screening and providing in vivo validation, these models bridge the gap between lab research and clinical application. A notable case involving a patient with triple-negative breast cancer demonstrated the real-time clinical utility of these models, identifying an FDA-approved drug that led to a complete response and significantly extended progression-free survival.5 This innovative approach underscores the potential of organoid technology to revolutionize precision oncology and drug development for breast cancer.5
Are you ready to share your groundbreaking research with healthbook TIMES Oncology Hematology?
It’s evident that this groundbreaking technology will drive significant advancements in oncology, marking an exciting era for the field. The transformative impact of organoid technology, particularly for older patients, is immense. This innovative method is set to revolutionize personalized cancer treatment by tackling its complexities with unmatched precision. We warmly invite researchers to share their pioneering discoveries in this area with healthbook TIMES Oncology Hematology. As an open-access, peer-reviewed journal indexed in Scopus, EuroPub and Google Scholar, and listed in the Directory of Open Access Journals (DOAJ) and the Registry of Open Access Resources (ROAD), we offer a prestigious platform for your research. Our membership with the World Association of Medical Editors (WAME) guarantees extensive visibility and accessibility, with research securely archived in CLOCKSS. We sincerely thank all authors, peer reviewers and readers for their invaluable contributions and look forward to continued collaboration and progress in oncology and hematology throughout 2024 and beyond.
Sincerely,
PD Dr Marcus Vetter
Chief Physician
Head of Center Oncology & Hematology
Cantonal Hospital Baselland (KSBL)
Liestal, Switzerland
marcus.vetter@ksbl.ch
Conflict of interest
The author received honoraria for consultancy from GSK, Gilead, Roche, Novartis, Exact Sciences, Pfizer, AstraZeneca, Daiichi Sankyo, Stemline, AbbVie and ASC Oncology. These funding entities did not play a role in the development of the manuscript and did not influence its content in any way.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.