Introduction
In non-small cell lung cancer (NSCLC), common deletions in exon 19 (Ex19del) and L858R mutations in exon 21 comprise approximately 90% of all epidermal growth factor receptor (EGFR) mutations. These are the most prevalent driver mutations accessible for palliative first-line therapy and should be particularly tested in lung adenocarcinomas. The prevalence of EGFR mutations varies globally from 14.1% in Europe to 38.4% in China. These mutations occur more often in females and are mainly detected in NSCLC among never smokers.1 In contrast, EGFR exon 20 insertion (Ex20ins) mutations are relatively rare and account for up to 12% of EGFR mutations, ranking third in frequency.2–5
Generally, due to the multitude of available treatment options for NSCLC and the increasing understanding of the interactions of certain mutations, such as TP53, STK11 and KEAP1, with common therapies in palliative stages, comprehensive next-generation sequencing (NGS) is already recommended at the initial stage, particularly for adenocarcinomas. EGFR Ex20ins mutations constitute a molecularly heterogeneous group with more than 100 variants and are identified through NGS using tumor DNA.6–8
Primarily driven by the high prevalence of these EGFR mutations in NSCLC, there exists a high medical demand for treatment optimization, prompting substantial research efforts aimed at optimizing therapy options for these patients. In this review, I highlight the key findings from recent studies in the field of EGFR mutations.
Current treatment landscape in the metastatic and locally advanced first-line setting for common EGFR mutations
The current standard therapy of choice for patients harboring common EGFR mutations (EGFRcm) such as Ex19del and L858R, or exhibiting EGFR T790M resistance mutations, as per findings from the FLAURA study, entails oral administration of the third-generation tyrosine kinase inhibitor (TKI) osimertinib.9–11
In the FLAURA study,12 osimertinib as first-line therapy demonstrated a significant extension in progression-free survival (PFS) compared with erlotinib or gefitinib (18.9 months vs 10.2 months; HR: 0.46) and overall survival (OS) (38.6 months vs 31.8 months; HR: 0.80; p=0.046). Overall, osimertinib exhibits excellent therapeutic tolerability, primarily manifesting as gastrointestinal and cutaneous toxicities. Despite those excellent response rates and the favorable safety profile, the majority of patients undergoing osimertinib monotherapy will relapse, and resistance is difficult to treat.13
New data in locally advanced and metastatic first-line setting
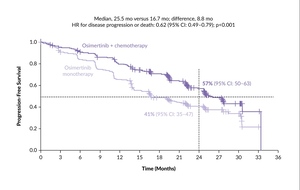
During the World Conference on Lung Cancer (WCLC) 2023, results from the FLAURA2 trial were presented.12 This study randomized patients with EGFRcm-positive NSCLC to receive either osimertinib combined with pemetrexed and platinum-based chemotherapy or osimertinib alone as frontline therapy. The trial enrolled 557 patients. The results demonstrated a clear advantage in PFS for the osimertinib plus chemotherapy combination compared with osimertinib monotherapy, as assessed by the investigators (HR for disease progression or death: 0.62 [95% CI: 0.49–0.79]; p<0.001) (Figure 1). By the 24-month mark, 57% of the patients in the osimertinib plus chemotherapy group and 41% in the osimertinib-only group remained free from disease progression. A blinded independent central review confirmed these findings (PFS HR: 0.62 [95% CI: 0.48–0.80]). Moreover, combination therapy yielded a higher objective response rate (ORR), with 83% of patients showing a complete or partial response compared with 76% in the osimertinib monotherapy group. The median duration of response (DoR) was notably longer with combination therapy, reaching 24.0 months versus 15.3 months with osimertinib alone. Of particular significance was the benefit observed in patients with central nervous system (CNS) metastases at baseline, where the median PFS was extended to 24.9 months with osimertinib plus chemotherapy versus 13.8 months with osimertinib monotherapy. Despite these promising outcomes, it is important to acknowledge the higher incidence of toxicity in the combination arm, primarily due to chemotherapy-related adverse events (AEs). Serious AEs were reported in 38% of patients receiving osimertinib plus chemotherapy compared with 19% in the osimertinib-only group. Consequently, discontinuation of osimertinib occurred in 11% and 6% of patients in the respective groups.12
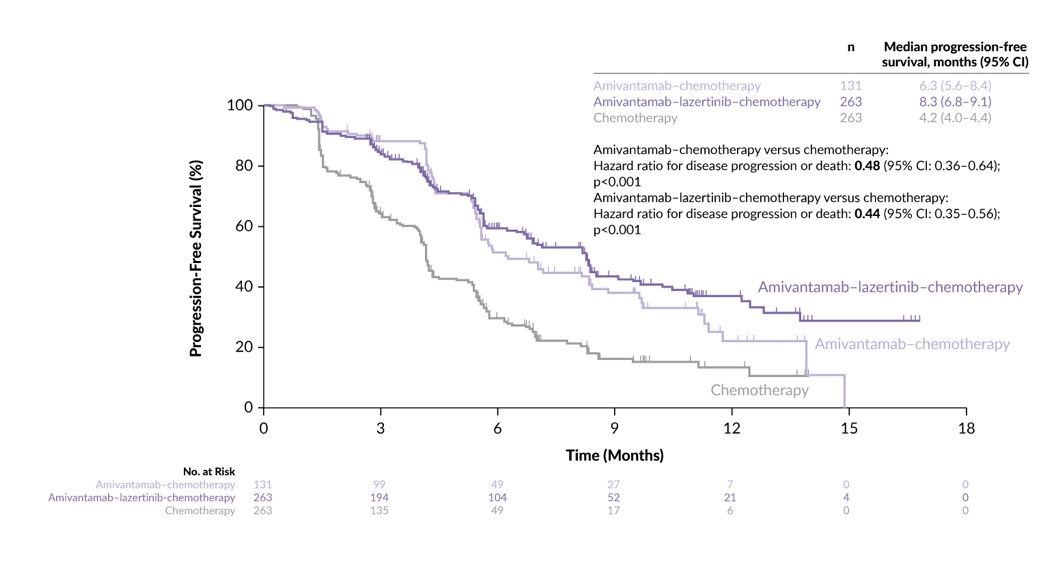
Another important new study is the MARIPOSA trial, which was unveiled at the European Society for Medical Oncology (ESMO) Annual Meeting 2023. This trial evaluated the efficacy of amivantamab, an EGFR-MET bispecific antibody, in combination with lazertinib, a third-generation EGFR TKI, compared with osimertinib in the first-line setting.14,15 Patients diagnosed with EGFRcm locally advanced or metastatic NSCLC were randomized at a 2:2:1 ratio to receive either amivantamab plus lazertinib, osimertinib alone or lazertinib alone. At a median follow-up of 22.0 months, the amivantamab plus lazertinib combination demonstrated a clinically meaningful improvement in PFS of 23.7 months compared with 16.6 months with osimertinib alone (Figure 2). Additionally, patients in the experimental arm exhibited a longer DoR of 25.8 months versus 16.8 months in the osimertinib arm, along with a favorable trend in OS (HR: 0.80 [95% CI: 0.61–1.05]; p=0.1). However, it is noteworthy that toxicity was more pronounced in the amivantamab plus lazertinib arm. Grade ≥3 gastrointestinal AEs in the combination arm included diarrhea (2%), stomatitis (1%), decreased appetite (1%) and nausea (1%). Among other frequent AEs were rash, pruritus, paronychia, acneiform dermatitis, peripheral edema, fatigue, infusion-related reactions and venous thromboembolism. Most amivantamab-related toxicities emerged within the first four months of treatment, underscoring the importance of interdisciplinary toxicity management, especially in collaboration with dermatologists, and implementing thromboprophylaxis strategies.14,15
At the European Lung Cancer Congress (ELCC) 2024, it was shown that approximately half of all patients in the amivantamab plus lazertinib arm had a dose interruption with a median time to dose interruption of six weeks and a median interruption duration of 22 days.16 Interestingly, amivantamab interruption did not appear to impact PFS.
In summary, both studies presented potentially new options in the first-line setting. However, careful consideration is required when determining the appropriate therapeutic regimen, as both the MARIPOSA and FLAURA2 regimens exhibit higher toxicity levels compared with osimertinib monotherapy. Subgroup analyses of the FLAURA2 study particularly demonstrate a benefit of combined chemotherapy plus TKI treatment in patients with CNS metastases. It is imperative to accumulate clinical experience regarding the side effects of amivantamab (with or without lazertinib) in everyday practice. Importantly, dermatological side effects necessitate close collaboration with colleagues in dermatology and vigilant monitoring of side effects during the initial months of treatment. Notably, infusion reactions related to amivantamab occurred in nearly all cases within the first 60 minutes, with treatment discontinuation required in only a small number of cases.
To improve tolerability and reduce administration time, a subcutaneous formula of amivantamab is currently being investigated. In the recently presented PALOMA-3 study, it was shown to be non-inferior to the intravenous administration in terms of ORR and pharmacokinetics. Furthermore, it also caused fewer side effects, especially fewer infusion reactions (13% vs 66%) and less venous thromboembolism (9% vs 14%) compared with intravenous administration.17
At the American Society of Clinical Oncology (ASCO) 2023 Congress, further analysis of the CHRYSALIS-2 study revealed that MET positivity by immunohistochemistry (IHC) may serve as a predictive biomarker for the response to amivantamab plus lazertinib in the post-osimertinib chemotherapy-naïve setting.18 This raises the question of whether patients with high baseline MET expression could particularly benefit from treatment with amivantamab plus lazertinib in the first line.
In the future, if approved, various first-line treatment options will pose a challenge in determining which patients are suitable for an intensified treatment regimen. In my opinion, given the good tolerability, excellent response rates and lower financial toxicity of osimertinib monotherapy, most patients will likely continue to be treated with osimertinib alone. However, for patients with a high and/or symptomatic tumor burden, young and fit patients aiming for a deep and long-lasting response and patients with brain metastases, an intensified treatment regimen may be justified. Moreover, a longer follow-up of the MARIPOSA and FLAURA2 trials will reveal whether initial intensified treatment will have a positive impact on OS.
What is new in the second and later lines?
Following progression on osimertinib monotherapy, heterogeneous mechanisms of acquired resistance can be observed. Approximately 13% of cases exhibit corresponding on-target resistances, such as C797X mutations or EGFR amplifications. In approximately 25% of cases, bypass resistance mechanisms are observed, including MET amplifications (METamp) in 15% of cases, HER2 amplifications in 2% of cases, as well as rare KRAS, PIK3CA, PTEN, BRAF, AKT or ERK mutations. Additionally, histological transformations into small cell lung carcinomas are observed in 5–15% of cases, and transformations into squamous cell carcinomas are observed in 15% of cases. The cause of progression remains unknown in approximately 50% of cases.19–23 Whenever possible, getting a new tissue biopsy of a progressing lesion is therefore very important in order to target the second-line treatment. In the following section, I will present some of the latest data in the second-line setting.
HERTHENA-Lung01 is a phase II trial evaluating the HER3-targeted antibody-drug conjugate (ADC) patritumab deruxtecan (Patri-DXd) in patients with advanced EGFR-mutant NSCLC who were previously treated with EGFR TKI therapy and platinum-based chemotherapy.24 Of 225 enrolled patients, 209 received a third-generation TKI. At study inclusion, 115 patients presented with CNS metastases, whereas 105 patients did not. The overall disease control rate was 73.8%, with a DoR of 6.4 months and a median OS of 11.9 months. Notably, among patients with CNS metastases, the median OS was 11.6 months compared with 12.9 months in patients without CNS metastases. Furthermore, 10 out of 30 patients with baseline CNS metastases and no prior radiotherapy demonstrated an intracranial response, indicating the efficacy of Patri-DXd in the CNS. AEs were mainly hematological toxicity and gastrointestinal toxicity contributed to the chemotherapy payload. Similar to other ADCs, adjudicated drug-related interstitial lung disease (ILD) was observed in 5.3% of patients (grade 5, 0.4%). The results of the ongoing phase III HERTHENA-Lung02 trial evaluating Patri-DXd versus platinum-based chemotherapy25 will be presented in the future.
Furthermore, subgroup analysis of the phase III TROPION-Lung01 trial, which investigated the ADC datopotamab deruxtecan (Dato-DXd) targeting TROP2, showed a favorable PFS compared with docetaxel in previously treated NSCLC patients with actionable genomic alterations.26,27
In the phase II TROPION-Lung05 trial, Dato-DXd was administered after at least one specific targeted therapy in patients with actionable genomic alterations (EGFR, ALK, ROS1, NTRK, BRAF, MET exon 14 skipping or RET).27 Of 137 patients, 56.7% had an EGFR mutation. The study results revealed a very good disease control rate of 78.8%, with a median DoR of 7 months. The most common AEs associated with this ADC are stomatitis, nausea and other gastrointestinal side effects, as well as ocular side effects, anemia and in some cases ILD. More phase III trial data on a higher number of patients with actionable genomic alterations would be interesting for this promising drug in this setting.
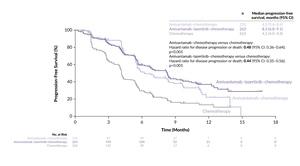
The combination of amivantamab and lazertinib was also evaluated in the second-line setting. In the phase III MARIPOSA-2 study, 657 patients with typical EGFR mutations after progression on osimertinib were randomized at a 2:2:1 ratio to receive amivantamab plus lazertinib plus chemotherapy, chemotherapy alone or amivantamab plus chemotherapy.28 This study demonstrated a significantly higher ORR for the amivantamab-containing regimens compared with chemotherapy alone (64% and 63% vs 36%, respectively; p<0.001 for both groups). Additionally, a PFS benefit was observed for the amivantamab-containing groups compared with chemotherapy alone, with median PFS of 8.2 and 6.3 months versus 4.2 months (Figure 3). Translation of these findings to OS benefit is eagerly anticipated. Intriguingly, the median intracranial PFS was extended to 12.5 and 12.8 months for the amivantamab plus chemotherapy and amivantamab plus lazertinib plus chemotherapy groups, respectively, compared with 8.3 months for chemotherapy alone. Lazertinib was initially added to the amivantamab plus chemotherapy arm in order to achieve a higher CNS response but interestingly, the addition of lazertinib did not lead to a difference in CNS outcome. AEs were more common in the amivantamab-containing arms, with grade 3 AEs reported in 48% for chemotherapy alone, 72% for amivantamab plus chemotherapy and 92% for amivantamab plus lazertinib plus chemotherapy. Skin toxicity, hematological toxicity and venous thromboembolism were particularly common AEs with the amivantamab-containing regimen. Furthermore, it would be intriguing to explore whether specific subgroups, especially those with MET overexpression, derive greater benefit from amivantamab-containing regimens.
Novel options in targeting MET as a resistance mechanism to osimertinib
Given that METamp and overexpression are the most common acquired resistance mechanism to osimertinib, are there already data available for the feasibility to combine EGFR and MET inhibitors in patients with these mutations?
The phase II INSIGHT 2 study assessed the efficacy of tepotinib in combination with osimertinib in advanced/metastatic EGFR-mutant NSCLC with acquired resistance to first-line osimertinib and METamp, determined centrally by fluorescence in situ hybridization (gene copy number [GCN] ≥5 and/or MET/CEP7 ≥2) and/or liquid biopsy NGS (MET plasma GCN ≥2.3). Of 128 patients that received tepotinib and osimertinib, 98 had a FISH METamp and showed a promising ORR of 50%, the median DoR of 8.5 months and the median PFS of 5.6 months.29,30 The promising results of this chemotherapy-sparing regimen present a very valuable treatment option in the subgroup of EGFRcm patients with METamp as a resistance mechanism to osimertinib.
The phase II SAVANNAH study demonstrated the efficacy of savolitinib, a highly selective MET TKI, when administered in combination with osimertinib in patients exhibiting high levels of MET overexpression and/or amplification.31 These patients were characterized by an IHC90+ and/or FISH10+ status and had experienced disease progression on osimertinib treatment. Among the 108 patients included in the study, the combination therapy achieved an ORR of 49%, accompanied by a median DoR of 9.3 months.
Building upon these promising outcomes, the ongoing phase III SAFFRON study is currently enrolling patients to evaluate the efficacy of the savolitinib plus osimertinib combination in comparison to platinum plus pemetrexed chemotherapy in this specific population.32
What about PD-(L)1-targeting immunotherapy (IO) in patients with EGFR mutations?
The administration of programmed cell death-1/programmed death-ligand 1 (PD-[L]1)- targeting agents in NSCLC with EGFR genomic alterations failed in many studies. We know from previous studies that IO monotherapy is not effective: in CheckMate-057 trial, subgroup analyses did not show PFS or OS benefit in patients with EGFR mutations. Similarly, the phase III KEYNOTE-010 and OAK trials did not show that patients with NSCLC harboring EGFR mutations had OS benefit from IO over chemotherapy.33–35
Looking at the subgroup analysis in the pivotal PACIFIC trial, in which durvalumab was administered for one year after definitive radiochemotherapy in locally advanced NSCLC, PFS and OS outcomes with durvalumab were similar to those with placebo in patients with EGFR-mutant tumors.36 It is suggested that different immunosuppressive mechanisms, including an altered tumor microenvironment, are involved in resistance to immunotherapy in EGFR-mutant NSCLC.
Regarding the combination of chemotherapy plus IO, the CheckMate 722 study, which compared the combination of nivolumab plus platinum-based chemotherapy to chemotherapy only after progression on EGFR TKIs, demonstrated no significant improvement in PFS.32 Similar negative PFS and OS benefits could be seen in the KEYNOTE-789 study which compared pembrolizumab versus placebo plus chemotherapy.37
Does adding anti-VEGF targeting provide any benefit to IO with or without chemotherapy in second and further treatment lines?
Anti-vascular endothelial growth factor (VEGF) agents in combination with PD-(L)1 targeting might increase the effect, with a potential synergistic immunomodulatory effect of anti-VEGF and IO therapy. However, there are conflicting data regarding the use of chemotherapy plus IO and VEGF targeting for EGFR mutations. The globally conducted IMpower150 study investigated atezolizumab plus carboplatin plus paclitaxel (ACP), bevacizumab plus carboplatin plus paclitaxel (BCP) or atezolizumab plus BCP (ABCP) every three weeks for four or six cycles, followed by maintenance therapy with atezolizumab, bevacizumab or both.38 A total of 35 patients with EGFR mutations received ABCP. The OS benefits for ABCP versus BCP in patients with sensitizing EGFR mutations (HR: 0.6), including those with previous TKI failure (HR: 0.74), were shown. ACP did not have survival benefit versus BCP in sensitizing EGFR mutations (HR: 1.0) or liver metastases (HR: 1.01).
In the Chinese phase III ORIENT-31 study, PD-1 targeted therapy with sintilimab in combination with platinum and pemetrexed chemotherapy with or without anti-VEGF targeting with the bevacizumab biosimilar IBI305 showed a benefit in PFS for chemotherapy in combination with IO with or without anti VEGF therapy. Sintilimab plus chemotherapy also significantly improved PFS compared with chemotherapy alone (median, 5.5 months vs 4.3 months; HR: 0.72; p=0.016). The PFS for anti-VEGF plus IO plus chemotherapy was 7.2 months, therefore showing a benefit over chemotherapy (HR: 0.51; p<0.0001).39
On the other hand, the Chinese phase III IMpower151 study, in which ABCP versus BCP was evaluated in a mixed population, failed to show a clinically relevant PFS benefit in the subgroup of patients with EGFR/ALK-positive disease (n=163).40 The median PFS was 8.5 months with ABCP versus 8.3 months with the BCP (HR: 0.86).
The phase III ATTLAS study, which was conducted in South Korea, compared ABCP plus pemetrexed maintenance (n=215) versus pemetrexed and platinum (PC) (n=74). Of 228 patients, 215 had an EGFR mutation and all patients were pretreated with a TKI. The median OS was not significantly different between the groups (20.63 months vs 20.27 months; HR: 1.01; p=0.975), even though the median PFS was significantly better in the ABCP arm than in the PC arm (8.48 months vs 5.62 months, HR: 0.62; p=0.004).41
In summary, considering the available and conflicting study results with all their limitations, a combined chemotherapy plus anti-VEGF and IO regimen can be considered for EGFRcm patients with progression after osimertinib, even though several factors such as increased toxicity, higher costs, longer infusion times and controversial data on efficacy over chemotherapy alone have to be considered and discussed with our patients. For many patients, platinum-based chemotherapy doublet without IO remains the most feasible option.
Current data in localized disease in NSCLC with common EGFR mutations
The data from the ADAURA study have already made their way into clinical practice, demonstrating a significant benefit favoring adjuvant treatment with osimertinib compared with placebo over three years in patients with stage II–IIIA disease.42 An 83% reduction in the risk of disease recurrence or death with the use of adjuvant osimertinib compared with placebo was observed (HR: 0.17 [99.06% CI: 0.11–0.26]; p<0.001). At ASCO 2023, the 5-year OS data were presented, revealing a 5-year OS rate of 85% in the osimertinib group (n=233) compared with 73% in the placebo group (n=237) (HR: 0.49; p=0.0004) (Figure 4).43,44 The HR for CNS disease-free survival (DFS) in stage II–IIIA disease was 0.24 (95% CI: 0.14–0.42). These positive OS data confirm the rationale for the use of osimertinib in the adjuvant setting. At the ASCO meeting this year, minimal residual disease (MRD) analysis from the ADAURA trial showed that patients with MRD negativity rarely relapse during adjuvant treatment, making it a very good prognostic factor for DFS.45 It was discussed that MRD positivity, which often preceded a relapse with a median time of 4.7 months before the event, could possibly identify patients who might benefit from a longer adjuvant treatment.
The phase III NeoADAURA study, which recently completed recruitment, aimed to assess the efficacy of neoadjuvant osimertinib with or without chemotherapy compared with chemotherapy alone prior to surgery in patients diagnosed with resectable stage II-IIIB N2 EGFR mutation-positive NSCLC.46 In the phase IIb NEOS trial, which included 88 patients with stage IIA-IIIB (T3-4 N2) disease and EGFRcm, patients received osimertinib for six weeks. The ORR was 71.1% in the 38 patients that completed the treatment; therefore, targeting EGFR in the neoadjuvant setting might become an option in the future.47
At this year’s ASCO meeting, data from the phase III LAURA trial were presented. This trial included 216 patients with unresectable stage III EGFR-mutant NSCLC, whose disease remained stable following definitive platinum-based chemoradiotherapy.48 Patients received osimertinib (n=143) or placebo (n=73) until disease progression, unacceptable toxicity or meeting other discontinuation criteria. At the data cut-off, patients in the experimental arm showed a significantly longer PFS of 39.1 months versus 5.6 months (p<0.001) (Figure 5).49,50 In the osimertinib group, a higher toxicity were observed versus placebo with grade ≥3 AEs reported in 35% versus 12% of patients, respectively, especially with a higher rate of radiation pneumonitis (48% vs 38%) diarrhea (36% vs 14%) and rash (24 vs 14%). Interstitial lung disease was reported in 8% of patients in the osimertinib arm, most of them grade 1 or 2. The OS data of this study will be crucial in determining whether the very promising PFS benefit will translate into an OS advantage and confirm if implementing osimertinib therapy after definitive chemoradiotherapy is the right approach.
What is new in metastatic NSCLC with EGFR Ex20ins mutations?
The current ESMO guidelines recommend a platinum-based chemotherapy doublet with or without immune checkpoint inhibitors for patients with EGFR exon 20 mutations.9 In this disease setting, the bispecific EGFR-MET-targeting antibody amivantamab demonstrates remarkable efficacy, as evidenced by findings from the CHRYSALIS and PAPILLON trials.
Last year, updated data from the phase I CHYSALIS study were presented at the ELCC. In this study, pretreated patients with Ex20ins were treated with amivantamab, resulting in an ORR of 37% among a total of 114 patients, with a median DoR of 12.5 months.51,52 The median PFS was 6.9 months. No new safety signals were identified, and rash (89%), infusion-related reactions (67%) and paronychia (58%) remained the most common treatment-emergent AEs. The median OS was 23 months. This treatment option for Ex20ins addresses an unmet need and represents an effective therapeutic option. It has been approved as second-line therapy in Switzerland since September 2022.
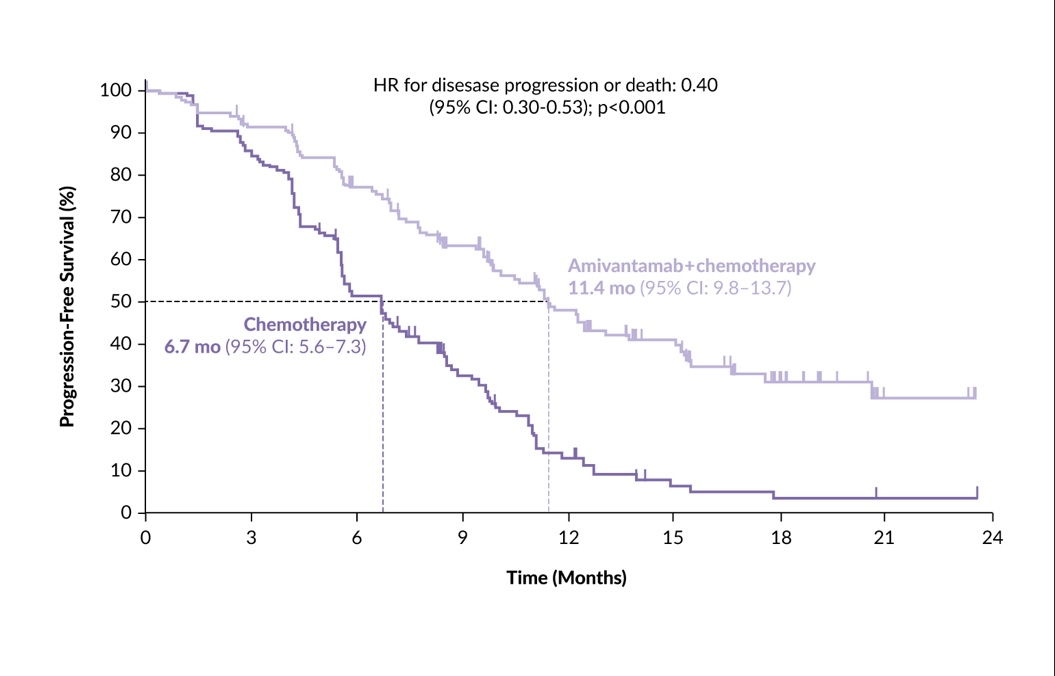
In the first-line setting, the recently published phase III PAPILLON trial assessed the efficacy of amivantamab plus chemotherapy versus chemotherapy alone.53 Among 308 patients, PFS was notably longer in the amivantamab plus chemotherapy group compared with the chemotherapy group (median 11.4 months vs 6.7 months; HR for disease progression or death: 0.40 [95% CI: 0.30–0.53]; p<0.001) (Figure 6). In the interim OS analysis (with 33% data maturity), the HR for death with amivantamab plus chemotherapy versus chemotherapy was 0.67 (95% CI: 0.42–1.09); p=0.11. No new safety signals occurred for any agent, and treatment discontinuation occurred in 7% of patients.
Another agent that showed clinical activity in patients with EGFR Ex20ins mutations is mobocertinib. Based on findings of a phase I/II trial, in which mobocertinib in pretreated patients showed an ORR of 28% with a DoR of 17.5 months and a median OS of 24 months,54 the EXCLAIM-2 trial was conducted. In this phase III study, mobocertinib was tested in the first-line setting versus platinum-doublet chemotherapy.55 Similar PFS (median, 9.59 months with mobocertinib vs 9.63 months with chemotherapy), as well as a comparable ORR (32% vs 30%) between the two arms, were observed. Because these results of EXCLAIM-2 did not show a prespecified PFS benefit over chemotherapy, mobocertinib was withdrawn from the market by the company. Among other oral TKIs that are currently being evaluated in patients harboring EGFR Ex20ins mutations and show promising results are poziotinib,56 sunvozertinib57 and zipalertinib.58
Treatment possibilities for other uncommon EGFR mutations
ACHILLES is a Japanese phase II study involving 109 patients with uncommon EGFR mutations (G719X, L861Q, S768I and compound mutations).59 At a median follow-up of 12.5 months, PFS was significantly longer in the afatinib arm compared with platinum doublet chemotherapy (10.6 months vs 5.7 months; HR: 0.422; p=0.0007), with an ORR of 61.4% versus 47.1%, respectively. While these results suggest a benefit of afatinib over chemotherapy, caution is needed in interpreting these findings and applying them to clinical practice due to the variety of mutations and the small number of patients involved. The study examined many different stratification factors, making it numerically underpowered. The Swiss Group for Clinical Cancer Research (SAKK) LuCa study, an observational study including patients with atypical EGFR mutations in one cohort, aims to provide more insights in this area.60 Whenever possible, patients with rare EGFR mutations should be presented at a molecular tumor board for comprehensive evaluation.
Conclusion
In conclusion, it is evident that significant advancements are being made in the field of targeted therapies for NSCLC patients with EGFRcm. In the future, we will have a wider array of first-line treatment options available. Both intensified treatment regimens from the FLAURA2 and MARIPOSA studies for EGFRcm cases appear to be more effective compared with the highly potent osimertinib monotherapy. Moving forward, it will be crucial to determine which patients might benefit from more intensive treatment compared with the well-tolerated TKI monotherapy.
In subsequent lines of treatment, we also see promising developments with new ADCs and the combination of chemotherapy with amivantamab, as shown in the MARIPOSA2 study, demonstrating encouraging results and addressing unmet medical needs. Additionally, it is reassuring to see new and effective treatment options emerging for the rarer EGFR exon 20 mutations.
Conflict of interest
Dr Sebastian Kraus serves as principal investigator for AstraZeneca, Daiichi Sankyo and GSK and has received advisory board payments from Roche, Janssen, Servier, AstraZeneca and Sanofi, as well as travel grants from several pharmaceutical companies. The funding entities and sponsors of the author did not play a role in the development of the manuscript and did not influence its content in any way.
Funding
The author has declared that no financial support has been received from any organization for the submitted work.
Author contributions
The author has created and approved the final manuscript.
_in_the_mariposa_trial.jpeg)

_data_from_the_laura_trial.jpeg)


_in_the_mariposa_trial.jpeg)

_data_from_the_laura_trial.jpeg)