Roadmap to cure in early and advanced HER2-positive breast cancer
Patients with HER2-positive breast cancer were traditionally treated with intensive dose-dense chemotherapy. With the advent of targeted therapies, there has been a shift towards potentially reducing treatment intensity (de-escalation). This change is particularly relevant in adjuvant and neoadjuvant settings, where cure rates have improved. In operable disease, both neoadjuvant or adjuvant approaches play an important role in disease control. The neoadjuvant approach, which was initially used to reduce tumor size before surgery, also offers several advantages for systemic disease control, including options for personalized treatment de-escalation, with pathologically complete response (pCR) as a surrogate for improved survival. Key questions include whether chemotherapy or surgery can be omitted in certain cases. Prof. Christoph Thomssen, University Martin-Luther, Halle-Wittenberg, Germany presented a comprehensive overview of the significant milestones in the treatment of HER2-positive breast cancer.
The pharmacological approach to targeting HER2-positive breast cancer has involved two main strategies (Figure 1).1 The first is a humanized monoclonal antibody targeting the outer domain of HER2, with two key drugs, trastuzumab and pertuzumab. The second strategy involves tyrosine kinase inhibitors (TKIs) that block the tyrosine kinase domain and consequently suppress the signaling pathways. Several TKIs are now available on the market, including lapatinib, neratinib, tucatinib and pyrotinib.
Trastuzumab was assessed in combination with chemotherapy in a pivotal phase III clinical trial that demonstrated an overall survival (OS) benefit of five months versus chemotherapy alone (25.1 months vs 20.3 months; p=0.046).2 Data from the more recent phase III CLEOPATRA trial showed that the combination of trastuzumab, pertuzumab and docetaxel versus trastuzumab and docetaxel alone further prolonged OS (median, 56.5 months vs 40.8 months; HR: 0.68 [95% CI: 0.56−0.84]; p<0.001).3 Indeed, comparing results from older trials to those of newer trials reveals substantial OS improvements. Historically, treatment with chemotherapy alone led to a median OS of around 20 months and approximately 25 months with adding one antibody to chemotherapy. In contrast, the current standard of dual antibody blockade with trastuzumab and pertuzumab, combined with docetaxel, has extended median survival to 57 months, representing an absolute difference of nearly three years.
The next step in treatment development involved the use of designer antibodies, known as antibody-drug conjugates (ADCs), which are engineered to carry a payload of highly toxic chemotherapy. The first ADC targeting HER2 receptor was trastuzumab emtansine (T-DM1),4 which demonstrated significantly improved both progression-free survival (PFS) (median 9.6 months vs 6.4 months; HR: 0.65 [95% CI: 0.55−0.77]; p<0.001) and OS (median, 30.9 months vs 25.1 months; HR: 0.68 [95% CI: 0.55−0.85]; p<0.001) versus the standard combination lapatinib plus capecitabine in the second line of HER2-positive breast cancer.5 The next significant advancement was with the DESTINY-Breast03 trial.6 In this study, the next-generation ADC trastuzumab deruxtecan (T-DXd) significantly improved survival outcomes versus T-DM1 in patients with HER2-positive metastatic breast cancers (Figure 2).6
Tucatinib, a next-generation TKI, was introduced at almost the same time as T-DXd. In combination with trastuzumab and capecitabine, this agent demonstrated a significant PFS benefit in heavily pretreated patients with HER2-positive metastatic breast cancer (1-year PFS rate, 33.1% vs 12.3% with trastuzumab and capecitabine alone; HR: 0.54 [95% CI: 0.42−0.71]; p<0.001), which further translated into a significant OS improvement (2-year OS rate, 44.9% vs 26.6%; HR: 0.66 [95% CI: 0.50−0.88]; p=0.005).7 Notably, tucatinib showed similar antitumor activity in patients with brain metastases.
Adjuvant/neoadjuvant therapy for breast cancer
In the adjuvant setting, several phase III studies evaluating trastuzumab showed consistent results across various trials.8–12 For example, a combined analysis of NCCTG N9831 and NSABP B-3 revealed that adding trastuzumab to standard adjuvant chemotherapy reduced recurrences by nearly half (p<0.001) and significantly decreased death rate by almost 40% (p<0.001) in patients with operable HER2-positive breast cancer.8 Similar improvements with adjuvant trastuzumab were shown in HERA, BCIRG-006, finHER and PACS-04 studies.9–12
Both neoadjuvant and adjuvant treatments aim to achieve systemic control and many key studies have explored these approaches. In the prospective, phase II TECHNO study, patients with HER2-overexpressing breast cancer received epirubicin and cyclophosphamide followed by paclitaxel plus trastuzumab as neoadjuvant treatment; trastuzumab was continued after surgery to complete one year of treatment.13 Results showed that survival outcomes were associated with the pCR status. Specifically, the 3-year disease-free survival (DFS) rate was 88% in patients with pCR compared with 73% in patients without pCR (p=0.01) and the 3-year OS rate was 96% and 86%, respectively (p=0.025). Several key questions arose from these data, including whether enhancing the pCR rate improves clinical outcomes and whether additional therapy is needed based on pCR status.
The phase II, proof-of-concept NeoSphere trial evaluated the preliminary efficacy of single or double HER2 blockade, with and without a taxane, in the neoadjuvant setting. Results showed that adding pertuzumab to the combination of trastuzumab and docetaxel nearly doubled the pCR rate.14,15 The 5-year PFS rates were 86% for the double blockade and 81% for the single blockade (p=0.0141), with some effect seen even with trastuzumab and pertuzumab alone (73%).15 These results support the use of double HER2 blockade with trastuzumab plus pertuzumab and chemotherapy in patients with operable HER2-positive breast cancer in the neoadjuvant setting.
Patients without pCR have a worse prognosis than those who achieve pCR. The phase III KATHERINE trial aimed to assess the efficacy of T-DM1 versus trastuzumab in patients with residual disease after receiving neoadjuvant therapy containing a taxane and trastuzumab.16,17 The T-DM1 arm showed 11.3% fewer invasive DFS events compared with the trastuzumab arm (88.3% vs 77.0%; HR: 0.50 [95% CI: 0.39−0.64]; p<0.001). Long-term data demonstrated a 13.7% reduction in invasive DFS events at a median follow-up of 8.4 years, with a hazard ratio of 0.54 (95% CI: 0.44−0.66; p<0.0001).18 This translated into an OS rate of 89.1% with T-DM1 and 84.4% with trastuzumab in the second interim analysis (HR: 0.66 [95% CI: 0.51−0.87]; p=0.0027).
Analyzing 20 years of data from a tumor bank revealed significant improvements in prognosis over time with HER2-directed therapy in early breast cancer.19 In detail, those patients who were treated between 2000 and 2004 without anti-HER2 treatment had an unfavorable prognosis in terms of invasive DFS and OS. The availability of trastuzumab between 2005 and 2011 resulted in a considerably better prognosis. After 2011, the use of more sophisticated treatment strategies, including dual blockade with trastuzumab and pertuzumab, and the use of T-DM1 in cases of non-pCR, have resulted in significantly improved OS.
Adjuvant and neoadjuvant therapy in HER2-positive breast cancer: a critical reappraisal
During the meeting, Dr Andreas Gerteis, Breast Cancer Center, Hospital Robert Bosch, Stuttgart, Germany discussed de-escalation regiments and approaches to chemotherapy-free treatment. Traditionally, treatment for HER2-positive breast cancer has involved intensive regimens combining surgery, chemotherapy and targeted therapies such as trastuzumab and pertuzumab. However, de-escalation strategies have emerged to reduce treatment burden and minimize toxicity without compromising efficacy. Results from randomized clinical trials showed that a proportion of patients with breast cancer can achieve pCR with chemotherapy-free treatment. Specifically, in the phase II WSG-ADAPT HER2+/HR- trial, 34.4% of patients with early breast cancer receiving 12 weeks of neoadjuvant trastuzumab plus pertuzumab had a pCR.20 The same treatment regimen was investigated in early breast cancer patients with hormone receptor (HR)-positive, HER2-positive tumors in the prospective WSG-TP-II trial, demonstrating a pCR rate of 25% in the chemotherapy-free arm.21
De-escalated chemotherapy in early HER2-positive breast cancer
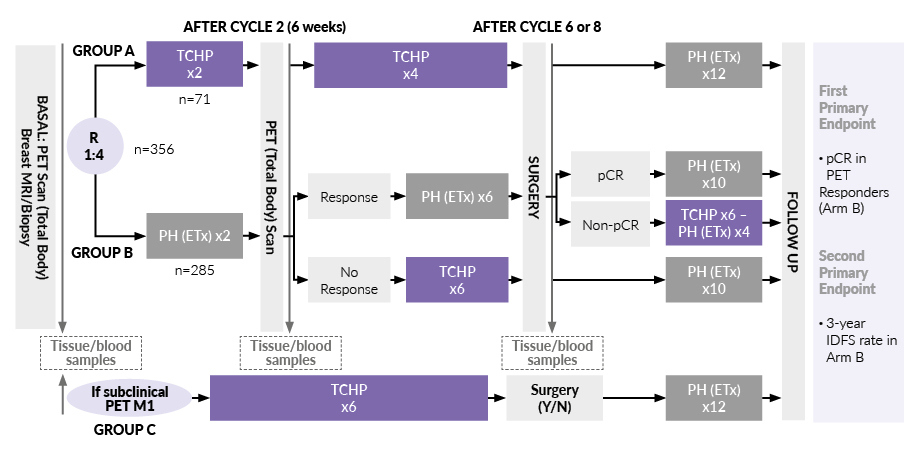
A neoadjuvant chemotherapy-free regimen with a response-adapted strategy was further explored in the PHERGain trial in patients with HER2-positive early breast cancer based on early metabolic response by 18F-FDG positron emission tomography (PET)/computerized tomography (CT) to neoadjuvant trastuzumab plus pertuzumab and the pathologic response.22,23 The design of the study is presented in Figure 3. Overall, 79.6% of patients who received two courses of trastuzumab plus pertuzumab (group B) were PET responders. The primary endpoint of the pCR rate in this subset of patients in group B was 37.9% (p<0.001; null hypothesis, pCR ≤20%).23 The second primary endpoint of the 3-year invasive DFS rate in group B was also met (95.4%; p<0.001). In total, only 3% of patients in group B (n=8) experienced distant recurrences.
Building on the encouraging results from the PHERGain trial, several studies have explored the potential to predict a pCR with chemotherapy-free regimens. The PAMELA trial investigated the presence of stromal tumor-infiltrating lymphocytes (TILs) and intrinsic subtypes as predictive markers for pCR in HER2-positive early breast cancer treated with anti-HER2-based therapy, but this approach did not prove very effective.24 TBCRC023 was another notable study in this setting, examining two different time schedules, 12 weeks versus 24 weeks of trastuzumab plus lapatinib, in women with HER2-positive breast cancer.25 The study found that the pCR rate was not influenced by HR status, but patients with HR-positive tumors required more time to achieve pCR. Specifically, there was no considerable difference between 12- and 24-week dual blockade in HR-negative tumors in pCR rate (20% vs 18%), while in HR-positive tumors, the pCR rate increased from 12% after 12 weeks to 28% with extended treatment.
Considering these data, the question arose of whether postponing chemotherapy could harm patients. In group B of the PHERGain trial, 141 patients received 8 courses of dual blockade, with 50% not achieving pCR, which resulted in a 24-week delay of chemotherapy. However, this postponement of chemotherapy did not worsen therapeutic outcomes, as there was no significant difference between groups A and B in 3-year invasive DFS, 3-year distant disease-free survival (DDFS), event-free survival (EFS) and OS (Table 1). This is consistent with findings from other studies, such as the KRISTINE trial,26 where the 3-year invasive DFS rate was 94.2% with the treatment regimen consisting of docetaxel, carboplatin, trastuzumab and pertuzumab (TCHP), and the TRAIN II study,27 which showed similar 3-year EFS and OS rates for anthracycline-containing regimens. These comparisons suggest that delaying chemotherapy does not compromise long-term outcomes in patients who initially respond well to antibody treatments. It is important to note, however, that PHERGain and other studies mentioned above had limited statistical power to make definitive conclusions; larger studies are needed to unequivocally demonstrate that the postponement of chemotherapy does not impact treatment outcomes.
Practical considerations of implementing chemotherapy-free regimens
When considering the implementation of the PHERGain treatment strategy, several factors come into play, especially regarding the integration of post-neoadjuvant treatment with T-DM1. For patients with HR-positive, HER2-positive tumors, longer therapy with pertuzumab and trastuzumab for eight courses over 24 weeks is beneficial, as shown in the PHERGain trial.23 In case of non-pCR, a biopsy should be performed at Week 24; if non-pCR is confirmed, chemotherapy with six courses of TCHP should be initiated, followed by surgery at Week 42. Patients with non-PCR can further receive T-DM1 for four cycles, bringing the total treatment duration to 54 weeks. However, it is not fully clear whether four cycles of T-DM1 are sufficient. Insights from the ADAPT HR+/Her2+ trial,28 which showed a pCR rate of 41% pCR with four cycles of T-DM1, and the KRISTINE trial,29 which demonstrated a pCR rate of 35% after six cycles of T-DM1 plus pertuzumab, suggest that most benefits from T-DM1 might be achieved within four cycles.
Another practical consideration is the impact of tumor HER2 expression status and tumor heterogeneity on the activity of chemotherapy-free treatment regimens. According to the findings from the WSG-TP-II trial, there were no pCR in HER2-low tumors (immunohistochemistry [IHC] score of 0−2) treated with endocrine therapy (ET) plus trastuzumab and pertuzumab, while the pCR rate was 25.0% in those with IHC score ≥3.21 In the chemotherapy-containing arm, these rates were 12.5% versus 59.8%, respectively. These results indicate that HR-positive tumors with HER2 IHC 2+/FISH-positive status are not the best candidates for chemotherapy-free treatment. In addition, the KRISTINE trial29 showed a decrease in efficacy within the first few months by approximately 8%, likely due to tumor heterogeneity. Another study further revealed that approximately 10% of HER2-positive tumors exhibit heterogeneity.30,31 In these heterogeneous tumors, no pCR was observed with T-DM1 plus pertuzumab compared with a pCR rate of 55% in non-heterogeneous tumors (p<0.001).
In conclusion, only phase II studies have so far been published regarding chemotherapy-free dual blockade. Data indicate that pre-treatment with dual blockade does not worsen therapy outcomes and that achieving pCR is associated with an excellent prognosis in patients with HER2-positive breast cancer.
Modern therapeutic approaches to brain metastases and leptomeningeal disease
Up to 50% of patients with HER2-positive metastatic breast cancer develop brain metastases despite advances in treatment.32 Although the survival of these patients has improved over time, recurrent central nervous system (CNS) events remain a major reason for morbidity and mortality, and effective therapies are lacking. During the meeting, Prof. Dr Christian Kurzeder presented the current treatment landscape for HER2-positive breast cancer patients with brain metastases, including leptomeningeal disease.
In the KATHERINE trial, patients treated with T-DM1 showed significantly improved survival outcomes compared with those treated with trastuzumab.16 However, CNS was a site of first recurrence in approximately 5% of patients in both treatment groups. The activity of T-DM1 was also assessed in patients with HER2-positive metastatic breast cancer and brain metastases in the single-arm phase IIIb KAMILLA study.33 In a post hoc analysis, T-DM1 demonstrated efficacy in this patient population, with an overall response rate (ORR) of 21.4% and a clinical benefit rate of 42.9%. A reduction in the sum of the major diameters of brain metastases of at least 30% occurred in 42.9% of patients. In patients with baseline brain metastases, the median PFS was 5.5 months and the median OS was 18.9 months. Furthermore, dual anti-HER2 blockade with pertuzumab and trastuzumab also demonstrated CNS activity. In a subset of patients who developed CNS metastasis during the CLEOPATRA trial,3 those receiving the pertuzumab-containing therapy had numerically longer OS compared with those receiving the placebo-containing therapy (median, 34.4 months vs 26.3 months; HR: 0.66 [95% CI: 0.39–1.11]; p=0.1139).34
Patients with HER2-positive metastatic breast cancer whose disease progressed after multiple HER2-targeted agents have limited treatment options. In this patient setting, the randomized HER2CLIMB study investigated the addition of tucatinib, a highly selective inhibitor of the HER2 tyrosine kinase, to trastuzumab and capecitabine in patients who had previously been treated with trastuzumab, pertuzumab and T-DM1.7 An updated exploratory analysis showed that tucatinib prolonged OS for 9.1 months in patients with brain metastases (21.6 months vs 12.5 months; HR: 0.600 [95% CI: 0.444–0.811]; p=0.00078), with 24-month OS rates of 48.5% versus 25.1% (Figure 4).35 Among patients with active brain metastases, the median CNS-PFS was 9.6 months in the tucatinib-combination group and 4.0 months in the placebo-combination group, with an estimated 1-year CNS-PFS rate of 32.1% and 0%, respectively. Among those with stable brain metastases, the median CNS-PFS was 13.9 months in the tucatinib-combination group and 5.6 months in the placebo-combination group, with an estimated 1-year CNS-PFS rate of 52.7% and 30.0%, respectively.
Another agent investigated in patients with brain metastases has been T-DXd. In the phase III DESTINY-Breast-03 trial, this ADC demonstrated antitumor activity in patients with HER2-positive unresectable or metastatic breast cancer who have received at least one prior anti-HER2-based regimen, including those with brain metastases.36 A pooled analysis of the DESTINY-Breast01, -02 and -03 trials further confirmed the efficacy of T-DXd in this clinical setting, consistently demonstrating superior intracranial response rates with T-DXd versus comparator therapies.37 Among patients receiving T-DXd, the intracranial ORR was 45.2% in those with treated/stable brain metastases and 45.5% among those with untreated/active brain metastases; these percentages were 27.6% and 12% among patients receiving comparator therapy (Figure 5). T-DXd showed a trend towards prolonged CNS-PFS over the comparator, with a greater advantage in patients with untreated/active brain metastases (18.5 months vs 4 months) than in patients with treated/stable brain metastases (12.3 months vs 8.7 months).
The phase IIIb/IV DESTINY-Breast12 aimed to further evaluate T-DXd in approximately 500 patients with previously treated advanced/metastatic HER2-positive breast cancer, both with and without brain metastases.38 This study enrolled patients whose disease had progressed with at least one prior anti-HER2-based regimen and received up to two lines of therapy in the metastatic setting, excluding tucatinib. Primary endpoints include ORR in patients without brain metastases and PFS in patients with brain metastases. The findings of this trial will be presented at upcoming congresses.
Treatment beyond T-DXd
The current European Society for Medical Oncology (ESMO) living guidelines recommend T-DXd in the second-line setting for patients with no, unknown or stable brain metastases.39 Patients with active brain metastases and no need for local intervention should receive either tucatinib plus capecitabine and trastuzumab (preferred treatment option) or T-DXd. In the third-line treatment, both of these therapies play a significant role.40
While tucatinib plus capecitabine and trastuzumab are recommended in the third-line setting, the efficacy of this combination after T-DXd exposure has not been fully clear. A French multicenter retrospective study included patients with HER2-positive metastatic breast cancer who were treated with tucatinib plus capecitabine and trastuzumab after prior treatment with T-DXd.41 In the overall population, the median PFS was 4.7 months and the median OS was 13.4 months at a median follow-up of 11.6 months. Among patients with baseline metastases at the triplet initiation, the median PFS was 4.4 months compared with 5.0 months among patients without brain metastases.
T-DXd shows activity in leptomeningeal disease
Leptomeningeal disease is a devastating complication of advanced metastatic cancer, associated with a poor prognosis and limited treatment options.42 Between 5–15% of patients with metastatic breast cancer develop leptomeningeal metastases.43 Despite significant advances in the treatment of HER2-positive metastatic breast cancer, no therapies are specifically approved for leptomeningeal metastases. Recent data indicated T-DXd activity in patients with HER2-positive breast cancer and leptomeningeal metastases.
In a case series of eight patients with heavily pretreated HER2-positive metastatic breast cancer and progressing leptomeningeal metastases, all patients derived clinical benefit from T-DXd treatment, with four patients achieving an objective partial response.44 The median OS from the first cycle of T-DXd was 10.4 months, with six patients still alive at the data cut-off. The median OS from the diagnosis of leptomeningeal metastases was 15.5 months. Encouraging antitumor activity of T-DXd was also reported in a patient with brain and meningeal metastases and HER2-low breast cancer.45
Taken together, T-DXd and tucatinib are established therapies for patients with HER2-positive breast cancer and stable and active brain metastases. However, there is currently no standard of care for leptomeningeal disease.
Best sequencing strategies in HER2-positive and HER2 low advanced breast cancer: HR-positive and HR-negative disease
The selection of optimal treatment strategies in breast cancer depends on many factors, such as disease status and tumor load, the pace of disease, the presence of comorbidities and frailty, biomarker evaluations, the availability of the medication, the patient’s preferences and the findings from recent clinical trials. Furthermore, HER2 expression status is an important indicator for the treatment decision-making. During his presentation, PD Dr Marcus Vetter, Cantonal Hospital Baselland, Liestal, Switzerland, discussed optimal drug combinations and sequencing strategies in breast cancer with different levels of HER2 expression.
In the HER2-positive setting, the treatment landscape expanded rapidly over the past decades, with many new options now available, including antibodies, small molecule inhibitors, PI3K inhibitors, mTOR inhibitors, bispecific antibodies and ADCs, with novel drug combinations and sequencing increasingly used.1 The combination of trastuzumab, pertuzumab and docetaxel is now the frontline standard of care based on the results of the CLEOPATRA trial.3 In the second line, practice-changing data favoring T-DXd over T-DM1 were reported in the DESTINY-Breast03 trial.6 In the third line, tucatinib plus trastuzumab and capecitabine is now a preferred regimen,46 with T-DM1 representing another option. The advantage of adding tucatinib to trastuzumab plus capecitabine was demonstrated in the HER2CLIMB trial,7 with efficacy of tucatinib in the third line after T-DXd confirmed in the real-world studies.47–49 Chemotherapy plus TKI is available for patients with at least four prior lines of therapy. There has also been a significant change in treatment strategies for HR-positive and HER2-low disease (IHC1+, IHC2+/ISH−), which is a subgroup within the HER2-negative population (IHC0, IHC1+, IHC2+/ISH−). Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors including ribociclib,50 palbociclib51 and abemaciclib52 are used in combination with endocrine therapy in the front line46; the combination of CDK4/6 inhibitors with PI3K inhibitors shows promising outcomes in HR-positive, HER2-negative PIK3CA-mutated locally advanced or metastatic breast cancer.53 In the second line, the optimal treatment sequence is not well defined and research is ongoing.
Later options also include chemotherapy, ADCs such as T-DXd and sacituzumab govitecan, as well as poly(ADP-ribose) polymerase (PARP) inhibitors in patients with BRCA1/2 mutations. In patients with disease resistant to endocrine therapy where mutations in the ESR1 gene play the major role,54 PFS with the second-line regimens remains generally low, ranging from 1.9 months to 11 months. Several drugs are under development in this setting, including selective estrogen receptor degrader (SERDS). In the phase III EMERALD-1 study, oral SERD elacestrant demonstrated a significant PFS improvement versus standard of care both in the overall population of patients with HR-positive, HER2-negative advanced breast cancer and in patients with ESR1 mutations.55 The phase II SERENA-2 study showed a statistically significant and clinically meaningful PFS benefit with camizestrant over fulvestrant in patients with advanced HR-positive, HER2-negative disease after recurrence or progression after up to one line of endocrine therapy.56 A new AKT inhibitor capivasertib in combination with fulvestrant significantly prolonged PFS versus fulvestrant alone among patients with HR-positive advanced breast cancer with mutations in the AKT pathway whose disease had progressed during or after previous aromatase inhibitor therapy with or without a CDK4/6 inhibitor.57
Another important option is T-DXd which demonstrated beneficial PFS and OS after one line of therapy in the HER2-low disease in the phase III DESTINY-Breast04 trial.58 In the phase II DAISY study, T-DXd further achieved confirmed ORRs of 70.6%, 37.5% and 29.7% in patients with HER2-overexpressing (HER2 IHC 3+ or ERBB2 ISH+) (n=68), HER2-low (HER2 IHC 2+/ERBB2 ISH– or IHC 1+) (n=72) and HER2-non-expressing (HER2 IHC 0) (n=37) metastatic breast cancer, respectively.59 Finally, the first-in-class Trop-2-targeting ADC sacituzumab govitecan demonstrated OS benefit in HR-positive, HER2-negative metastatic breast cancer in the phase III TROPiCS-02 trial (HR: 0.79 [95% CI: 0.65–0.96]; p=0.02).60 Many other treatments targeting HR pathway in HR-positive, HER2-negative breast cancer are expected to emerge in the coming years.61
In metastatic triple-negative breast cancer (TNBC), the first-line standard of care is represented by chemotherapy in combination with immunotherapy using immune checkpoint inhibitors, such as pembrolizumab or atezolizumab.62 In relapsed or refractory metastatic TNBC, sacituzumab govitecan was the first approved ADC based on the results from the phase III ASCENT trial which demonstrated improved PFS (HR: 0.41 [95% CI: 0.32–0.52]; p<0.001) and OS (HR: 0.48 [95% CI: 0.38–0.59]; p<0.001) compared with single-agent chemotherapy among patients without brain metastases, positioning sacituzumab govitecan as preferred option in the second-line setting.63,64 Other options in the second line include PARP inhibitors in patients with BRCA mutations. In the subset analysis of the DESTINY-Breast04 trial, PFS and OS benefits with T-DXd were evident also in a small population of patients with HER2-low, estrogen receptor (ER)-negative (IHC 0%) (n=58) and ER-low (IHC 1−10%) (n=52) tumors after one or two previous lines of chemotherapy, with high ORR being observed regardless of ER expression, positioning T-DXd as a preferred option in the third-line setting for the HER2-low subpopulation.65
The ABC of ADCs: Considerations on toxicity management
In the final lecture, Dr Anton Oseledchyk, University Hospital Basel, Basel, Switzerland, discussed safety of ADCs, with the focus on comparing toxicity profiled of T-DXd and T-DM1. Treatment of breast cancer with ADCs is generally associated with significant toxicity,66 with various adverse events (AEs) linked to different agents. Both the monoclonal antibody and cytotoxic payload parts of ADC molecules contribute to drug-related toxicity. The primary distinctions between T-DXd and T-DM1 molecules include their payload (topoisomerase I inhibitor vs anti-microtubule agent), drug-to-antibody ratio (8:1 vs 3.5:1), as well as the presence of a tumor-selective cleavable linker and evidence of a bystander anti-tumor effect with T-DXd.67 These features of T-DXd result in a substantial improvement in survival rates compared to T-DM1, as demonstrated in the DESTINY-Breast03 trial,6 but also lead to increased toxicity rates.
In a systematic review and meta-analysis of 169 clinical trials, the overall incidence of treatment-related AEs in patients treated with ADCs was 91.2% for all-grade events and 46.1% for grade ≥3 events.68 Among all ADCs, T-DXd was the agent most commonly causing all-grade AEs (98.0%), and it was also among the top ten ADCs causing grade ≥3 events (51.6%). The rate of AEs with T-DM1 was 95% for all-grade events and 33.1% for grade ≥3 events. Toxicity of T-DXd is typical for the topoisomerase I inhibitor payload, with the most frequent AEs being nausea (any grade, 73.5%; grade ≥3, 6.6%)69 which requires administration of antiemetics in most of the patients, as well as interstitial lung disease (ILD)/pneumonitis. T-DM1 exhibited a toxicity profile characteristic of anti-microtubule agents, with the most common AEs being thrombocytopenia (all grade, 52.5%; grade ≥3, 24.9%), as well as elevated liver enzymes and peripheral neuropathy.69 Although the overall incidence of toxicity is higher with T-DXd, it is important to note that a direct comparison is not entirely accurate in this context. In DESTINY-Breast03, treatment-emergent AEs (TEAEs) leading to treatment interruption were more prevalent with T-DXd compared to T-DM1 (52.9% vs 29.1%).70 However, the median treatment duration was also substantially longer with T-DXd (18.2 months vs 6.9 months with T-DM1), which may have played a role in the higher incidence of TEAEs with T-DXd. The overall rates of grade ≥3 drug-related TEAEs were similar between T-DXd (56.4%) and T-DM1 (51.7%). The most common TEAEs associated with treatment discontinuation were pneumonitis (5.8%), ILD (5.1%) and pneumonia (1.9%) with T-DXd, compared with decreased platelet count (1.5%), pneumonitis (1.1%) and thrombocytopenia (1.1%) with T-DM1.70
Lung toxicity, specifically ILD and pneumonitis, is an AE of special interest associated with anti-HER2 ADC therapy. In the DESTINY-Breast01 trial, five fatal cases of ILD were reported with T-DXd in patients with HER2-positive metastatic breast cancer previously treated with T-DM1.71 However, no adjudicated drug-related ILD/pneumonitis of grade 4–5 were observed in later trials.70 Severe cases of ILD are very rare, as long as regular chest computed tomography (CT) scans are performed, and lower-grade ILD is manageable with current treatment guidelines. As demonstrated in a recent analysis of the DESTINY-Breast04 trial, among six patients with grade 1 ILD/pneumonitis who were retreated with T-DXd after resolution, only one patient had a second ILD/pneumonitis event that was adjudicated as grade 2.72 Alopecia is another common AE associated with T-DXd, with an incidence rate of 36.2% among patients in the DESTINY-Breast03 population, with the majority of cases (26%) being grade 1.69 To mitigate the risk of alopecia, it is recommended to use scalp cooling for 20−45 minutes before and until 20−150 minutes after the infusion. Regarding cardiac toxicity, the incidence of decreased left ventricular ejection fraction (LVEF) was low (all grade, 2.7%; grade ≥3, 0%) in DESTINY-03 Breast.73 The guidelines for cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS)74 provide recommendations for surveillance in low-, moderate- and high-risk patients receiving HER2-targeted therapy, including electrocardiography (ECG), transthoracic echocardiography (TTE) and cardiac biomarker analysis.
Conclusion
Recent advancements in the treatment of HER2-positive breast cancer have been marked by the development of novel HER2-targeted therapies, including monoclonal antibodies and ADCs, as well as the implementation of combination therapies that enhance treatment efficacy. Future directions for ADC therapy in HER2-overexpressing breast cancer, as well as in HER2-low disease, are aimed at evaluating novel drug combinations and sequencing strategies, optimizing current chemotherapy regiments, as well as exploring novel emerging agents with improved efficacy and safety profiles. With ongoing innovations and a deeper understanding of tumor biology, HER2-targeted therapy stands at the forefront of personalized medicine, offering hope for better management and improved survival for patients with HER2-positive breast cancer.
Conflict of interest
PD Dr Marcus Vetter received honoraria for consultancy from GSK, Gilead, Roche, Novartis, Exact Sciences, Pfizer, AstraZeneca, Daiichi Sankyo, Stemline, AbbVie and ASC Oncology. Prof. Christian Kurzeder received honoraria from Tesaro, GSK, Astra Zeneca, Novartis, PharmaMar, Genomic Health, Roche, Eli Lilly S.A., Pfizer, Daiichi Sankyo, honoraria for consultancy or advisory role for Tesaro, GSK, Astra Zeneca, Novartis, PharmaMar, Genomic Health, Roche, Eli Lilly S.A., Merck MSD, Pfizer, and travel grants from GSK, Astra Zeneca and Roche.
Funding
Preparation of this article was supported by the Daiichi Sankyo – AstraZeneca Alliance and Gilead. The supporting companies did not have any decision-making role in the development of the manuscript and did not influence its content in any way.
Author contributions
All authors contributed to and approved the final manuscript.
-posi.png)
_progression-free_survival_by_blinded_independent_central_revi.jpeg)
_in_patients_with_brain_metastases_in_the_her2climb_study.jpg)
_in_pooled_analysis_of_destiny-breast01__-02_and_-.jpg)
-posi.png)
_progression-free_survival_by_blinded_independent_central_revi.jpeg)
_in_patients_with_brain_metastases_in_the_her2climb_study.jpg)
_in_pooled_analysis_of_destiny-breast01__-02_and_-.jpg)