Introduction
The European Chimeric Antigen Receptor (CAR) T-cell Meeting, jointly organized by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT), is a growing annual scientific conference that took place this year for the sixth time in Valencia, Spain. Gathering esteemed experts, researchers and clinicians from across the globe, the meeting provided a dynamic forum for the exchange of groundbreaking research and innovative insights to its 1,200 international attendees. Addressing physicians and basic and translational researchers, including specialized sessions for nurses, the meeting covered a wide array of aspects related to CAR T-cell treatment and development. Key themes included the pivotal role of CAR T-cell therapy in hematologic malignancies, with special sessions on multiple myeloma (MM) and acute leukemia, alongside its potential in new frontiers, such as solid tumors and autoimmune diseases. Compared with previous editions, concentrating on pivotal phase II and III trials, this year’s meeting presented real-world data on lymphoma therapy, e.g., confirming the efficacy of CD19-targeting therapy for transformed follicular lymphoma.1 Furthermore, discussions focused on optimizing manufacturing processes, overcoming challenges related to resistance and toxicity management, and addressing regulatory considerations to enhance accessibility. To date, six CAR T-cell products are approved worldwide, including in Switzerland.
Lymphodepletion
CAR T-cell therapies have significantly improved the outcomes of patients with various forms of B-cell non-Hodgkin lymphoma (NHL), B-cell acute lymphoblastic leukemia (B-ALL) and MM. Unfortunately, a substantial proportion of patients eventually relapse.
Lymphodepletion is used in different schemes (most commonly fludarabine [Flu] and cyclophosphamide [Cy]) and varying doses, as presented at the meeting by Prof. Ulrich Jäger of the Medical University of Vienna and recently reviewed by the T2EVOLVE Work Package 6 members,2 creating the opportunity to investigate its impact on toxicities as well as clinical outcomes. Lymphodepletion has three key roles: (1) reducing endogenous lymphocytes to create a conducive environment for CAR T-cell engraftment, expansion and long-term persistence; (2) decreasing tumor burden to prevent CAR T-cell exhaustion; and (3) preparing and modifying the tumor microenvironment and soluble factors to facilitate optimal CAR T-cell homing and viability/activity.2 Hence, agents with cytoreductive effects on both tumor cells and T cells are commonly used, based on conditioning regiments from trials of tumor-infiltrating lymphocytes and allogeneic hematopoietic cell transplantation (HCT).2,3 Flu and Cy are most frequently used, with total doses ranging from 75−120 mg/m2 for Flu and 750−1,500 mg/m2 for Cy.2 While the optimal dose remains unclear, higher-intensity lymphodepletion, although associated with increased toxicity, seems to improve CAR T-cell expansion, persistence and efficacy.2,4
While most commercially available and academic products use Flu/Cy, the JULIET trial on tisagenlecleucel included bendamustine as an alternative option for patients with previous resistance to a Cy-containing regimen or severe hemorrhagic cystitis after Cy.5 A post-hoc analysis on this subgroup (n=22; 20%) suggested a longer progression-free survival (PFS) for Flu/Cy than bendamustine but the analysis was not adjusted for differences in patient characteristics.6 In contrast, a larger retrospective study on lymphodepletion before tisagenlecleucel found that in matched cohorts, bendamustine had similar efficacy as Flu/Cy.7 In this study, a total of 90 patients (68.2%) received bendamustine as the lymphodepleting regimen and 42 patients (31.8%) received Flu/Cy, based on physician preference and institutional practice. Most noteworthy, bendamustine was associated with significantly less toxicity, including cytokine release syndrome (CRS), neurotoxicity, hematological toxicities, infections and reduced hospital readmissions. However, more studies on optimal regimens are necessary, as highlighted by the T2EVOLVE consortium.
Early-line CAR T-cell therapy for multiple myeloma
Since the first approval of B-cell maturation antigen (BCMA)-directed therapies idecabtagene vicleucel (ide-cel) in March 2021 and ciltacabtagene autoleucel (cilta-cel) in February 2022, CAR T-cell therapy is revolutionizing the field of MM. Initially approved after at least four prior lines, CAR T-cell therapy is now moving towards earlier lines of treatment. Based on the results from the phase III KarMMa-3 (NCT03651128)8 and CARTITUDE-4 (NCT04181827) trials,9 which were reviewed at the meeting by Prof. Hermann Einsele, the United States Food and Drug Administration (FDA) recently (05.04.2024) extended the approval for the use of ide-cel after two or more prior lines of therapy (including an immunomodulatory drug [IMiD], a proteasome inhibitor [PI] and an anti-CD38 monoclonal antibody) and cilta-cel after only one prior line of therapy (including a PI and an IMiD and in patients who are refractory to lenalidomide).
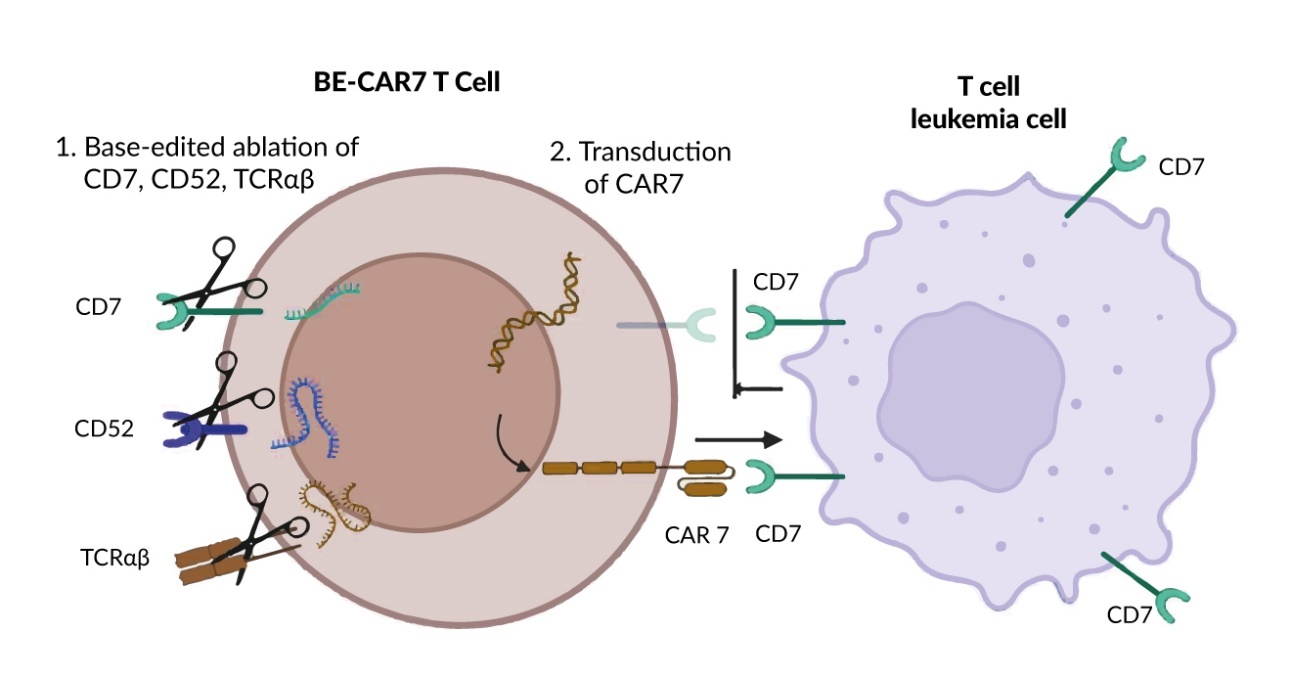
In the CARTITUDE-4 study, 208 patients with relapsed and lenalidomide-refractory MM and one to three previous lines of therapy received cilta-cel and 211 received standard of care (SoC). At a median follow-up of 15.9 months (range, 0.1–27.3), the median PFS was 11.8 months with standard regimens and was not yet reached with cilta-cel (HR: 0.26 [95% CI: 0.18–0.38]; p<0.001) (Figure 1).9
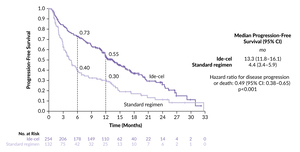
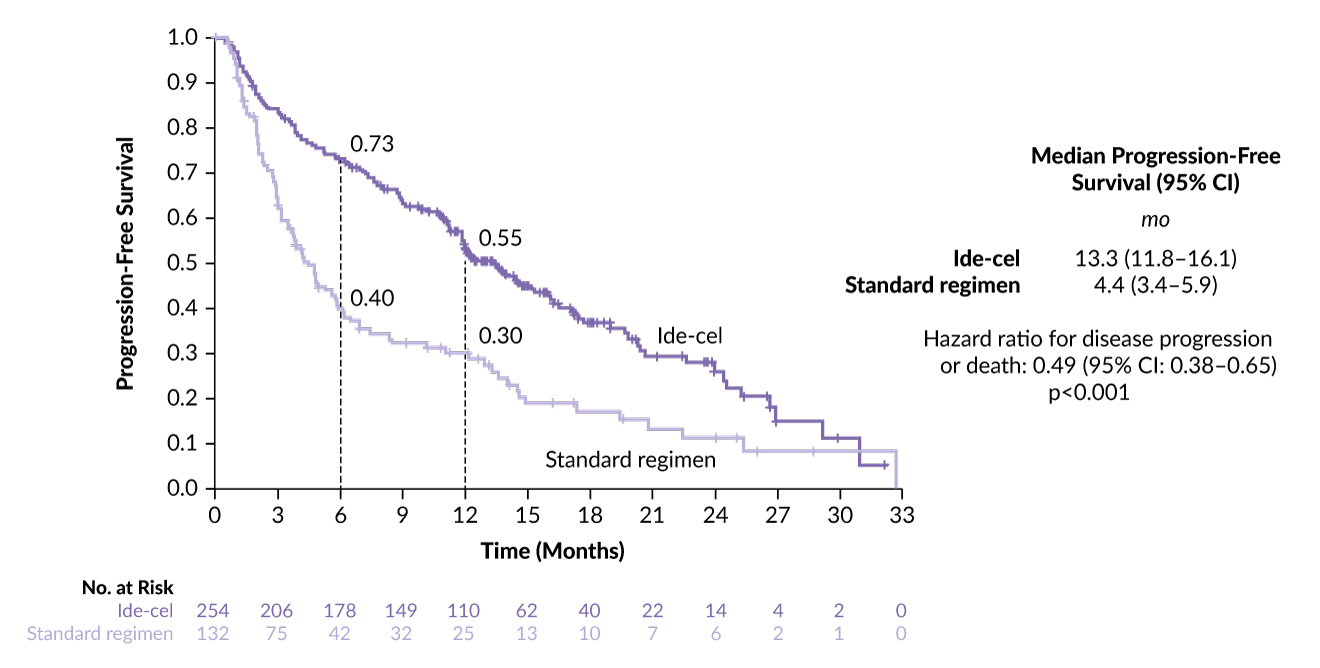
In KarMMa-3, ide-cel also demonstrated a significant improvement in PFS of 13.3 months (95% CI: 11.8–16.1) compared with 4.4 months in the standard-regimen group (HR: 0.49 [95% CI: 3.4–5.9]; p<0.0001) (Figure 2).8 In contrast to CARTITUDE-4, patients in the ide-cel group (n=254) and those in the SoC group (n=132) in KarMMa-3 were more heavily pretreated, with a median of two to four prior lines and 95% (vs 25% in CARTITUDE-4) were refractory to anti-CD38 antibodies.
Patients reported improved quality of life in both studies compared with the SoC. While PFS appeared similar in individual trials, a single-center real-world comparison, in which 35 (66%) patients received ide-cel and 18 (34%) patients received cilta-cel, suggested that cilta-cel might be superior in terms of efficacy.10 The median objective response rate (ORR) for cilta-cel was 94.4% versus 65.7% for ide-cel (p=0.01) and the median PFS for cilta-cel versus ide-cel was not reached (with a very short follow-up for cilta-cel) versus 10.9 months (p=0.09), respectively. However, the investigators of this retrospective analysis firmly acknowledged that the longer manufacturing time of cilta-cel may introduce a bias favoring the use of ide-cel for patients with rapidly progressing disease, potentially leading to inferior outcomes (Figure 3).10
As is already the case in lymphoma, CAR T cells will increase our armamentarium to fight MM in earlier treatment lines, with further exciting studies challenging the role of autologous stem cell transplantation (ASCT). In particular, centers in Switzerland will participate in the CARTITUDE-6 trial (NCT05257083), set to start in 2024, which aims to compare daratumumab, bortezomib, lenalidomide and dexamethasone (D-VRd) followed by cilta-cel versus ASCT in patients with newly diagnosed MM.
With a full session dedicated to MM, concluding with a bright look into the future, it also becomes clear that more data on the sequencing of therapy, including bispecific antibodies, are urgently needed. Despite new treatment approaches, the majority of patients with MM still relapse, prompting investigations into mechanisms of therapy escape and new targets, as vividly discussed in the meeting.
Mechanisms of resistance
A key tumor-intrinsic mechanism of resistance to immunotherapy is antigen escape, which has been studied for CD19 since the early clinical trials and is emerging recently as a factor in MM therapy.11 Dr Paola Neri from the University of Calgary presented a study on patients who had relapsed/refractory MM treated with anti-BCMA therapy (CAR T-cell therapy [n=5]; bispecific T-cell engager [TCE] therapy (n=16); both [n=3]). The team discovered a novel mechanism of BCMA antigenic escape involving functional epitope loss by a nontruncating mutation and in-frame deletions in the extracellular domain of BCMA encoded by the TNFRSF17 gene on chromosome 16p. In contrast to previous studies that found biallelic TNFRSF17 loss involving a large pre-existing chromosome 16p loss followed by a second focal genomic hit on the remaining allele, the team provided evidence that focal biallelic deletions of TNFRSF17 can also occur in the setting of a diploid chromosome 16p.12 Furthermore, functional analysis showed that some mutations, while maintaining detectable surface BCMA expression, inhibit the binding of anti-BCMA TCEs thereby abrogating their activity. These findings suggest that commonly used anti-BCMA antibodies in immunohistochemistry (IHC) or flow cytometry may not accurately predict anti-BCMA CAR T-cell/TCE resistance. Lastly, a higher rate of BCMA mutational events was found after TCE therapy compared with CAR T-cell therapy,12 potentially further supporting the earlier use of anti-BCMA CAR T-cell therapy prior to TCE.
Dr Marco Ruella provided an update on several further mechanisms of resistance to CAR-T cell therapy, which were recently reviewed.11 These include: (1) pre-infusion barriers such as low lymphocyte counts, progression during manufacturing and manufacturing failure; (2) tumor-intrinsic mechanisms, such as antigen loss, expression of inhibitory ligands, such as programmed death ligand 1 (PD-L1) and diminished apoptotic machinery; (3) CAR T-cell dysfunction including exhaustion; and (4) an immunosuppressive tumor microenvironment, including barrier and vascular factors, cytokines and metabolic factors.
To overcome these hurdles, novel targets (e.g., GPRC5D, SLAMF7 in MM) and strategies involving dual (bispecific) CARs (e.g., CD19 and BCMA, NCT06097455) were discussed. Furthermore, keynote lectures on innovative CAR T-cell engineering showcased novel co-stimulatory approaches, such as CD27, presented by Prof. Stanley Riddell,13 and Boolean-logic AND-gated CAR T cells, presented by Dr Robbie G. Majzner, targeting both leukemic and solid tumors in xenograft mouse models.14 Dr Pietro Genovese discussed innovative methods aiming to target novel tumor antigens regardless of shared antigen expression on hematopoietic stem/progenitor cells (HSPCs). Avoiding off-tumor toxicity, in a pre-clinical model, the group utilized base-editing of HSPCs before bone marrow transplantation to provide resistance of HSPCS to CAR T cells,15 as well as antibodies, opening new horizons for treatment opportunities.
New frontiers
In a session on solid tumors, which included discussions on novel engineering approaches in preclinical models, Dr Concetta Quintarelli presented a phase I–II clinical trial on young patients with relapsed or refractory high-risk neuroblastoma, the most common extracranial solid tumor in children, which expresses disialoganglioside GD2. In total, 27 heavily pretreated children received novel GD2-directed CAR T cells, with 17 having a response to the treatment.16 CRS occurred in 74% of patients but was mild in all patients except one. Treatment was concluded to be feasible and safe, with further studies ongoing.
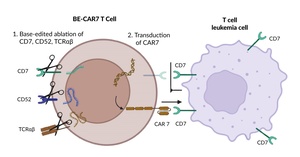
Combining revolutionary approaches in production and addressing an urgent medical need, Prof. Waseem Qasim presented the interim results of a phase I proof-of-principle study17 on base-edited allogeneic CAR T cells for relapsed T-cell ALL (T-ALL). Building upon CRISPR–Cas9 technology, which enables the exact targeting of specific genome sequences, base editing is a novel genetic engineering technique that allows for precise changes to individual DNA bases without causing double-stranded breaks and their associated risks. Utilizing this innovative technique, T cells from a healthy donor were edited in three genes before lentiviral transduction for an anti-CD7 CAR. The three highly precise edits inactivated the genes for (1) CD7 to evade CAR T-cell fratricide, preventing CAR T cells from targeting each other; (2) the (β chain of the) αβ T-cell receptor to prevent graft-versus-host disease; and (3) CD52 to evade anti-CD52-containing lymphodepleting conditioning (Figure 4). Pediatric patients in the study received lymphodepletion with Flu, Cy and alemtuzumab (anti-CD52), followed by infusion of base-edited (BE)-CAR7 T cells; if disease remission was achieved by day 28, patients underwent allogeneic HCT. At this point, persisting CAR T cells were depleted by the conditioning regimen. All three initial three patients, who had relapsed and refractory disease with no other curative options facing palliation for refractory leukemia, showed a response. One patient, with an initially high tumor burden of 80% blasts in the bone marrow, achieved morphologic and flow cytometric remission but had molecular persistence on day 25 and died on day 33 from infectious complications. Two other patients achieved molecular remission within 28 days after CAR T-cell infusion, underwent HCT and are still alive in remission. Infectious complications, a main concern in immune deficiency after serotherapy and CAR7 activity (by lymphodepletion, but also probably from anti-CD7 activity against other hematopoietic precursors), were manageable in two out of three patients. All patients had multilineage cytopenia, which has been noted in other CAR7 studies18 but was rescued by allogeneic HCT.17 With these encouraging results for refractory T-ALL, further data on the projected 10 patients in this study is awaited with interest. Furthermore, a similar study of “deep conditioning ahead of allogeneic HCT” is underway with a base-edited CAR against CD33 in children with acute myeloid leukemia (NCT05942599).
In the same session, Dr Corina Amor presented unique and intriguing preclinical findings on the potential of CAR T cells targeting senescent cells. The group had previously identified the urokinase-type plasminogen activator receptor (uPAR) as a cell-surface protein widely induced during senescence.19 They found that CAR T cells that target uPAR extend the survival of mice with lung adenocarcinoma and reverse liver fibrosis. Recently, they demonstrated that uPAR CAR T-cell treatment also improves exercise capacity in physiological aging and metabolic dysfunction in aged mice on a high-fat diet, both therapeutically and preventatively.20 When asked about the longevity of the mice, Dr Amor remarked that the mice stayed fit and lived long, but current studies are not sufficiently powered to draw robust conclusions yet.
A full session was dedicated to autoimmune diseases, a heterogeneous group of disorders where cellular therapies have recently emerged as a novel treatment approach. These are reflected in case reports, small case series and phase I studies.21 After an introduction, including the novel clinical practice guidelines from the EBMT practice harmonization and guidelines committee21 by Dr Raffaella Greco, Prof. Andreas Mackensen presented a groundbreaking case series published shortly after the meeting. In this study, 15 patients with severe autoimmune diseases (eight with systemic lupus erythematosus [SLE], three with idiopathic inflammatory myositis and four with systemic sclerosis) were treated with CD19 CAR T cells. All patients showed major clinical improvement and were able to stop immunosuppression. Within the median follow-up of 15 months, adverse events were minimal, with no high-grade CRS or immune effector cell-associated neurotoxicity syndrome (ICANS) and no prolonged bone marrow toxic effects.22
With a multitude of clinical trials ongoing, we look forward to the results of randomized studies and long-term outcomes. Of special interest for all further applications, especially for non-malignant diseases, is the risk of secondary malignancies. After the FDA’s recent report about T-cell malignancy following BCMA- or CD19-directed CAR T-cell therapies,23 this topic was also discussed among experts. Reporting on a large cohort of 449 patients treated at the University of Pennsylvania, Ghilardi and colleagues observed one case of T-cell lymphoma after axicabtagene ciloleucel (axi-cel) treatment with an overall projected 5-year cumulative incidence of 15% for solid and 2% for hematological malignancies.24 This was considered consistent with observations in patients treated with chemotherapy and/or radiation treatment, aligning with the FDA’s statement that the overall benefits of these products continue to outweigh their potential risks for their approved uses.23
Conclusion and outlook
In conclusion, the sixth European CAR T-cell Meeting highlighted significant advancements in CAR T-cell therapy, extending its impact from diverse hematologic malignancies to exploring new frontiers like solid tumors and autoimmune diseases. The seventh meeting will be held on February 6–8, 2025, in Strasbourg, France (and virtually), and promises to further drive collaboration and innovation, advancing transformative outcomes for patients globally.
Conflict of interest
Jana van den Berg received a travel grant by Gilead Sciences. The funding entity did not play a role in the development of the manuscript and did not influence its content in any way. Andreas Holbro has declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors have declared that no specific financial support was received for the submitted work.
Author contributions
All authors contributed to and approved the final manuscript.
_in_patients_with_lenalidomide-refractory.jpeg)
_with_idecabtagene_vicleucel_(ide.jpeg)
_in_patients_with_lenalidomide-refractory.jpeg)
_with_idecabtagene_vicleucel_(ide.jpeg)