Introduction
Endometrial cancer (EC) is the 15th most common cancer overall, the sixth most common cancer among women1 and the most prevalent gynecologic malignancy overall.2 EC predominantly affects post-menopausal women, with the incidence of early onset EC (women aged <50 years) being relatively rare.3 Based on data from 2022, the global age-standardized incidence and mortality rates are 8.7 cases and 1.8 cases per 100,000 people, respectively.1 In the US, the incidence is similar among most ethnic groups (approximately 28−31 new cases per 100,000 people), except for Asian/Pacific Islanders (24.5 new cases per 100,000 people); however, black women have almost double the age-adjusted mortality rate compared with other groups (9.3 deaths per 100,000 people). This is likely to be caused by several factors including histopathological, socioeconomic, cultural and treatment differences between ethnic groups.4,5 The growing prevalence of EC worldwide is a matter of concern, particularly in developed countries, where the increase is disproportionate.6 A possible rationale for the increase is the higher prevalence of certain risk factors among women (e.g., obesity, alcohol use, shifting reproductive trends, age, nulliparity, oral contraception, hormone replacement therapy and genetic factors such as Lynch syndrome).1,7–9 Preventative factors against EC include exercise, weight loss, pregnancy and diet (e.g., soy, coffee and tea intake) and are all associated with an inverse risk towards EC.10
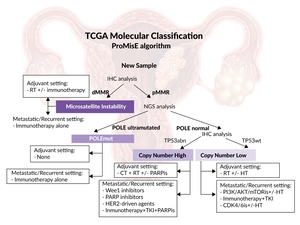
Historically, clinicopathological features (e.g., cancer stage, histologic grade, tumor subtype [endometrioid, serous, clear cell, mixed cell adenocarcinoma and other rare subtypes] and histopathologic markers) have been used for risk stratification of disease status in patients with EC.11,12 However, due to significant interobserver variability, more objective classification systems were developed.13,14 In 2013, The Cancer Genome Atlas (TCGA) project developed a system based on immunohistochemical and molecular markers to separate EC into four distinct prognostic subtypes.14 More recently, the Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) has been built based on the TCGA project and developed into a robust tool for assessing EC tumor subtype and associated risk (Figure 1).15,16 Following these advancements in clinical therapy, international guidelines for tumor classification and best-practice treatment have undergone significant changes to provide better outcomes for patients.17–19
Improvements in endometrial cancer classification
EC can be classified into different subtypes, based on their molecular characteristics. The initial four subgroups of EC identified were: POLE ultramutated (approximately 4%), microsatellite instability (MSI) hypermutated (approximately 39%), copy number low (approximately 49%) and copy number high (approximately 8%).14,20 The validated ProMisE system further developed the use of molecular subtyping by separating EC into four prognostically distinct subtypes: POLE-mutated (POLEmut), mismatch repair deficiency (dMMR), p53 wild-type (p53wt) and p53 abnormal (p53abn).16,21 In terms of incidence, POLEmut are present in 4–12% of EC tumors, more commonly in younger women with a lower body mass index, with early-stage disease, early onset of symptoms and very good prognosis22; dMMR tumors (23–36% of EC) are linked to lymphovascular space invasion (LVSI) (predictor of local and distant recurrence)23; p53abn tumors (8–24% of EC) are associated with higher stages of disease and poorest outcomes22,24; p53wt or no specific molecular profile (NSMP) tumors (30–60% of EC) are a heterogeneous group, primarily consisting of endometrioid subtype, and they express estrogen and progesterone receptors (ER/PRs).22 The 10-year analysis of cancer-related survival in the PORTEC-2 trial highlighted the prognostic value of molecular subtypes, with the highest survival among POLEmut subtypes, followed by NSMP, the dMMR subtypes and lastly p53abn subtypes (100%, 96.2%, 84.8% and 62.3%, respectively; p<0.001).24 The European Society for Medical Oncology (ESMO) has drafted recommendations for the classification of EC into risk groups (low-, intermediate- and high-risk).25 While there may be large overlaps with some ProMisE molecular subtypes (e.g., most p53abn cases are ESMO high risk and most POLE cases are low risk), they do not identify the same patients.21 Other factors that have been shown to have prognostic value include human epidermal growth factor receptor 2 (HER2), ER status and the presence of LVSI.26–28
The International Federation of Gynecology (FIGO)Iee on Women’s Cancer has recently updated the staging system to reflect new developments in our understanding of EC.17 Based on advancements in molecular profiling, the FIGO Committee encourages complete molecular classification (e.g., dMMR, NSMP and p53abn status) for all patients.
The molecular subtype can be recorded with the FIGO stage (e.g., stage IICmp53abn), and this profiling may upstage (p53abn) or downstage (POLEmut) disease status.17 Recent evidence from a pooled analysis of three European Society of Gynecological Oncology (ESGO)-accredited centers supports the updated 2023 FIGO staging system. In this analysis, the prognostic precision of the updated 2023 staging system was compared with that of the 2009 staging system in a cohort of patients.18 Patients identified as stage I using the 2023 system displayed a higher 5-year progression-free survival (PFS) rate compared to 2009 (93.0% vs 87.4%, respectively), while patients with stage III disease had a much poorer PFS rate (44.4% vs 54.1%, respectively). The 2023 staging system resulted in 27.6% staging shifts compared with the 2009 staging system (upshifts in 23.6% and downshifts in 3.9%).
Endometrial cancer staging
It is possible to stratify patients with EC according to risk and determine the optimal treatment option. In 2021, updated consensus guidelines on EC were provided by the ESGO, the European Society for Radiotherapy and Oncology (ESTRO) and the European Society of Pathology (ESP).19 These updated guidelines used FIGO staging, molecular classification and grading to stratify patients into the following risk groups: low, intermediate, high-intermediate, high and advanced/metastatic (Table 1).
Published in 2023, the updated FIGO staging of EC incorporates the latest pathology and molecular findings, providing an evidence-based system for EC staging.17 Significant updates to the FIGO staging system include the addition and refinement of substages within the FIGO system to represent the diversity and complexity of EC more accurately. Other changes are the adoption of risk stratification guidelines from the ESGO/ESTRO/ESP to improve prognosis and guide therapeutic decisions and the inclusion of molecular parameters to acknowledge their prognostic significance. The revision also provided a more comprehensive classification that incorporates histological types, tumor patterns and molecular characteristics to reflect a deeper understanding of the varied EC types.
Treatment modalities for patients with endometrial cancer
Several treatment modalities are available for EC patients, including surgery, chemotherapy, radiation therapy, hormone therapy and targeted therapies.
Surgery
Options include hysterectomy with bilateral salpingo-oophorectomy (BSO), sentinel lymph node (SNL) dissection or pelvic and periaortic lymph node dissection. Surgery is the primary treatment for EC, especially in early-stage disease.30
Radiation therapy
Patients may receive radiation therapy as adjuvant therapy following surgery to reduce the risk of local recurrence or as definitive treatment in cases where surgery is not feasible (e.g., vaginal brachytherapy, external-beam radiation therapy or stereotactic body radiation therapy [SBRT] for oligometastatic recurrences).31,32
Chemotherapy
In patients with advanced or metastatic EC or high-risk EC following surgery, chemotherapy may be recommended.30 Various treatment regimens are available; carboplatin and paclitaxel are preferred in advanced or recurrent EC, and although there is no standard of care (SoC) for second-line patients, evidence supports doxorubicin and paclitaxel as the most active treatment.19
Hormone therapy
For women with early-stage or advanced EC who are not surgical candidates or wish to preserve fertility, this may involve progestational agents (such as hydroxyprogesterone, medroxyprogesterone and megestrol), tamoxifen, aromatase inhibitors, cyclin-dependent kinase 4/6 (CDK4/6) inhibitors and combination therapies.33 Further development of hormone therapy is warranted as evidence suggests that, particularly in combination with other treatment regimens, it may benefit specific subgroups of patients in the first-line setting for metastatic or recurrent EC.34
Biologic and targeted therapies
Targeted therapies, including monoclonal antibodies and small-molecule inhibitors, are being investigated for the treatment of EC, particularly in cases of advanced or recurrent disease. Available therapeutics include mammalian target of rapamycin (mTOR) inhibitors, anti-vascular endothelial growth factor (VEGF) agents (e.g., bevacizumab, lenvatinib), HER2-targeted therapies and immune checkpoint inhibitors (ICIs) (e.g., pembrolizumab for high-frequency MSI [MSI-H] or dMMR tumors), nivolumab, dostarlimab and avelumab) either alone or in combination, including dual therapy and combination with chemotherapy.35,36
Patients should discuss their treatment options with a multidisciplinary team of healthcare providers, including gynecologic oncologists, radiation oncologists, medical oncologists and other specialists, to tailor a personalized treatment plan based on their circumstances.25
Current treatment guidelines for patients with advanced or metastatic endometrial cancer
While localized disease is curable with surgery (5-year survival rate of 95%), the prognosis for advanced or recurrent endometrial cancer remains poor, with 5-year overall survival (OS) rates of 20%.25,37 Current treatment options for patients with metastatic or recurrent EC in the first-line setting are limited, highlighting an unmet clinical need.
Treatment options for unresectable locally advanced disease include definitive radiotherapy or neoadjuvant chemotherapy followed by surgery or radiotherapy, with concurrent chemotherapy recommended to enhance the radiation effect.19 Residual lymph node disease after surgery should be treated with a combination of chemotherapy and external beam radiotherapy (EBRT) or chemotherapy alone to reduce the risk of distant metastases. Integrated or sequential boost and intensity-modulated radiation therapy (IMRT) should be used to minimize toxicity. Patients with residual pelvic disease after surgery should undergo individualized treatment with radiotherapy, chemotherapy or a combination therapy. In the case of oligometastatic disease radical local therapy should be considered.
For patients with recurrent EC, hormone therapy is the preferred initial systemic treatment for those with low-grade carcinomas without rapidly progressive disease. Progestogens such as medroxyprogesterone acetate and megestrol acetate are recommended; alternatively, aromatase inhibitors, tamoxifen or fulvestrant may be considered. The overall response rate (ORR) is up to 100% among patients with hormone receptor-positive disease in the first-line setting; however, responses are not typically seen in patients with hormone receptor-negative disease.34 Carboplatin and paclitaxel is the standard first-line chemotherapy,38 while doxorubicin and paclitaxel are commonly used in the second line.31 In patients with prolonged platinum-free intervals, re-introducing platinum-based therapy may be considered.19 Anti-programmed cell death protein 1 (PD-1) immunotherapy with pembrolizumab could be an option for second-line treatment of MSI/dMMR carcinomas, whereas a combination of pembrolizumab and lenvatinib might be considered for microsatellite stable carcinomas.19,31
Development of novel therapies for endometrial cancer is ongoing
As therapeutic options for patients with advanced or metastatic EC are limited, clinical research is ongoing to discover new and efficacious therapeutics. Novel therapeutics, such as mTOR inhibitors (e.g., everolimus and temsirolimus), antibody-drug conjugates (e.g., trastuzumab deruxtecan), poly (ADP-ribose) polymerase inhibitors (e.g., olaparib) and ICIs (e.g., pembrolizumab and avelumab), are being used alone and in combination across various clinical trials (Table 2). In particular, recent data from RUBY, NRG-GY018, AtTEnd and DUO-E have highlighted the potential for patient benefit with the combination of immunotherapy plus SoC chemotherapy.39–41 We will further discuss the results from these clinical trials in the next issue of the healthbook TIMES Oncology Hematology.
Conclusions
-
Our knowledge of the molecular characteristics of EC has evolved rapidly in recent years, with the FIGO staging guidelines being updated to better stratify patients into appropriate treatment pathways.
-
Novel monotherapy and combination therapies have shown promising results in patients with advanced or recurrent disease, who represent a difficult treatment population. In the next issue of healthbook TIMES Oncology Hematology, we will further discuss in detail recent updates on clinical trials investigating new approaches in EC therapy.
Conflict of interest
Prof. Matthew A. Powell received honoraria for consultancy from GSK, Merck, Eisai, AstraZeneca, SeaGen and Immunogen. PD Dr Marcus Vetter received honoraria for consultancy from GSK, Roche, Novartis, ExactSciences, Pfizer, Stemline, Abbievie and ASC Oncology.
Funding
The authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
All authors contributed to and approved the final manuscript.