Introduction
Cervical cancer is the fourth most deadly cancer in women globally, with an incidence of 7.7 per 100,000 women per year.1,2 Cervical carcinogenesis is primarily driven by human papillomavirus (HPV), a sexually transmitted virus with over 200 genotypes.3 Among these persistent infection with high-risk HPV types such as HPV-16 and HPV-18 poses the greatest risk for the development of cervical precancerous lesions and invasive cancer. High-resource countries have witnessed a substantial decline in cervical cancer incidence over the last decades, largely attributed to organized screening programs and HPV vaccination campaigns, while low-resource regions continue to be affected by a disproportionately high disease burden.4 Standard treatment options for cervical cancer include surgical interventions, chemo-/immunotherapy, radiotherapy and a combination thereof.5–7 In contrast to early-stage disease, recurrent or metastatic cervical cancer is associated with poor prognosis and a high mortality rate. Despite improved survival rates achieved with the introduction of the anti-angiogenic agent bevacizumab in the treatment regimen,8 most patients who experienced cancer progression after first-line platinum-containing chemotherapy have limited treatment options, accentuating the need for novel strategies for relapsed, recurrent or metastatic disease. Emerging targeted therapies, such as immune checkpoint inhibitors (ICIs) and antibody-drug conjugates (ADCs), offer promising avenues for advanced cervical cancer, with the potential to improve response rates and prolong survival. This review article discusses the current progress in cervical cancer therapy, with a focus on the most relevant and potentially practice-changing results of recent phase III clinical trials.
Immune checkpoint inhibitors
ICIs are cancer immunotherapies that target specific receptors on the surface of T-lymphocytes, leading to a powerful activation of immune responses.9 Programmed death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) are components of a crucial immune checkpoint pathway responsible for negative regulation of the stability and integrity of T-cell immune function. Importantly, PD-L1 upregulation has been observed in papillomavirus-associated cervical intraepithelial neoplasia10,11 suggesting the potential efficacy of the PD-1/PD-L1 blockade in cervical cancer treatment. Pembrolizumab, a highly selective humanized anti-PD-1 monoclonal antibody (mAb) that inhibits PD-1 binding to PD-L1, is a remarkable advancement in this field.12 Other promising ICIs include the PD-1-targeting mAb cemiplimab13,14 and anti-PD-L1 mAbs atezolizumab and durvalumab.
KEYNOTE-158: Pembrolizumab as monotherapy in advanced cervical cancer
KEYNOTE-158 is an international, open-label, multicohort, phase II basket study that evaluated pembrolizumab as monotherapy for several types of advanced solid tumors, including advanced cervical cancer.15,16 All patients received pembrolizumab for 2 years or until disease progression, intolerable toxicity, or physician or patient decision. The primary endpoint was objective response rate (ORR) assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) and the secondary endpoint was safety. The cervical cancer cohort included 98 patients (median age, 46 years). Among them, 78.6% had previously received ≥1 line of chemotherapy for recurrent or metastatic disease.
At a median follow-up of 36.9 months, pembrolizumab induced an overall response in 14.3% of patients (11/77) with PD-L1-positive tumors (combined positive score [CPS] ≥1) who had previously received at least one line of chemotherapy for recurrent or metastatic disease.15 Median progression-free survival (PFS) and overall survival (OS) were 2.1 months and 9.3 months, respectively. Treatment-related adverse events (TRAEs) occurred in 65.3% of the patients, including hypothyroidism (11.2%), fatigue (11.2%) and decreased appetite (9.2%). Grade 3–4 TRAEs occurred in 12.2% of patients; no grade 5 events were reported. In summary, pembrolizumab monotherapy demonstrated durable antitumor activity and manageable safety in patients with advanced cervical cancer. Based on these results, the US Food and Drug Administration (FDA) granted accelerated approval of pembrolizumab for patients with advanced PD-L1-positive (CPS ≥1) cervical cancer who experienced progression during or after chemotherapy.17
KEYNOTE-826: Pembrolizumab in combination with chemotherapy and bevacizumab in recurrent or metastatic cervical cancer
The randomized, double-blind, multicenter, phase III KEYNOTE-826 trial assessed the benefit of adding pembrolizumab to chemotherapy with or without bevacizumab in persistent, recurrent or metastatic cervical cancer.18 In total, 617 patients (age ≥18 years) with measurable disease per RECIST v1.1 and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0−1 were randomly assigned at a 1:1 ratio to receive pembrolizumab or placebo. All patients received platinum-based chemotherapy (paclitaxel plus either cisplatin or carboplatin); bevacizumab was included in the regimen according to the local practice per investigator’s discretion. The dual primary endpoints were PFS and OS.
The study demonstrated statistically significant and clinically meaningful PFS and OS improvements with the pembrolizumab-containing regimen compared with placebo. The final PFS and OS data with a median follow-up of 39.1 months were reported at the 2023 American Society of Clinical Oncology (ASCO) meeting19 and have recently been published in the Journal of Clinical Oncology.20 The median PFS was 10.5 months versus 8.2 months (HR: 0.58 [95% CI: 0.47–0.71]; p<0.0001) in patients with a PD-L1 CPS of ≥1, 10.4 months versus 8.2 months (HR: 0.61 [95% CI: 0.50–0.74]; p<0.0001) in the all-comers population and 10.4 months versus 8.1 months (HR: 0.52 [95% CI: 0.40–0.68]; p<0.0001) in patients with a PD-L1 CPS of ≥10 with pembrolizumab versus placebo, respectively. Median OS in the pembrolizumab group versus the placebo group were 28.6 months versus 16.5 months (HR: 0.60 [95% CI: 0.49–0.74]; p<0.0001), 26.4 months versus 16.8 months (HR: 0.63 [95% CI: 0.52–0.77]; p<0.0001) and 29.6 months versus 17.4 months (HR: 0.58 [95% CI: 0.44–0.78]; p<0.0001) in the PD-L1 CPS ≥1, ITT and CPS ≥10 populations, respectively (Figure 1).
The safety profile of the combination was consistent with previously reported results for the individual agents. The rates of grade ≥3 all-cause AEs and TRAEs were 82.4% versus 75.4% and 69.1% versus 65.0% in the pembrolizumab plus chemotherapy arm versus the placebo plus chemotherapy arm, respectively.19,20 The most frequent all-cause AEs were anemia (61.2% vs 54.0%), alopecia (56.4% vs 57.9%) and nausea (39.7% vs 43.7%) with pembrolizumab plus chemotherapy versus placebo plus chemotherapy, respectively. The analysis of patient-reported outcomes (PROs) using the European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life Core 30 questionnaire (QLQ-C30), EORTC cervical cancer module (QLQ-CX24) and EuroQol-5 dimension-5 level (EQ-5D-5L) visual analog scale demonstrated that the addition of pembrolizumab to chemotherapy with or without bevacizumab did not negatively affect health-related quality of life (HQoL),21 thus supporting the benefit of pembrolizumab in patients with recurrent, persistent or metastatic cervical cancer.
BEATcc: Atezolizumab plus chemotherapy and bevacizumab in advanced cervical cancer
The open-label, randomized, multicenter, phase III BEATcc academic study evaluated the PD-L1-targeting antibody atezolizumab combined with first-line platinum-based chemotherapy and bevacizumab in patients with advanced cervical cancer.22 The study enrolled women (≥18 years old) with recurrent, persistent or metastatic disease (stage IVB) not amenable to curative therapy. The participants had not received prior systemic therapy for relapsed/metastatic disease, had an ECOG PS score of 0 or 1 and were included irrespective of PD-L1 status. Stratification factors included prior concurrent chemoradiation, tumor histology and the chemotherapy backbone. A total of 410 patients were randomized 1:1 to receive atezolizumab plus doublet platinum chemotherapy (cisplatin or carboplatin plus paclitaxel) and bevacizumab or chemotherapy plus bevacizumab alone. The primary endpoints were PFS and OS and the key secondary endpoints included ORR, duration of response (DoR), time from randomization to first subsequent therapy or death (TFST), PFS to second progression or death (PFS2) and safety.
The primary results of the trial were reported at the ESMO Virtual Plenary Meeting 2023.23 At a median follow-up of 32.9 months, the addition of atezolizumab resulted in a significant improvement of both PFS (HR: 0.62 [95% CI: 0.49–0.78]; p<0.0001) (Figure 2) and OS (interim analysis HR: 0.68 [95% CI: 0.52–0.88]; p=0.0046). The median OS of 32.1 months and the ORR of 84% were observed in the atezolizumab arm compared to 22.8 months and 72% in the placebo arm, respectively. PFS and OS benefits were reported for all patient subgroups. With respect to safety, a predictable and manageable safety profile and no new safety signals were observed with atezolizumab in combination with chemotherapy and bevacizumab. There was no significant increase in toxicity in the atezolizumab arm compared with the control arm. Any-cause grade ≥3 AEs occurred in 79% of patients versus 75% with atezolizumab versus control, respectively, with grade ≥3 AEs of special interest (AESIs) for atezolizumab reported in 5% of patients.
In conclusion, this study confirmed the benefits of first-line combination therapy with ICIs, anti-angiogenic therapy and platinum-based chemotherapy for recurrent or metastatic cervical cancer.
KEYNOTE-A18: Pembrolizumab plus chemoradiotherapy in high-risk, locally advanced cervical cancer
The encouraging results of the phase III KEYNOTE-A18 trial that assessed the combination of pembrolizumab with concurrent chemoradiotherapy (CRT) in high-risk, locally advanced cervical cancer were recently presented at the European Society for Medical Oncology (ESMO) Congress 2023.24 This randomized, double-blind, global study enrolled 1,060 patients with newly diagnosed, previously untreated, high-risk, locally advanced cervical cancer (FIGO 2014 stage IB2−IIB with node-positive disease or stage III−IVA). Patients (age range, 22−87 years; median, 49 years) were randomized at a 1:1 ratio to receive 5 cycles of pembrolizumab (200 mg) plus concurrent CRT followed by 15 cycles of pembrolizumab (400 mg) or 5 cycles of placebo plus concurrent CRT followed by 15 cycles of placebo. More than 90% of patients had a PD-L1 CPS ≥1. Stratification factors included planned external beam radiotherapy type (intensity-modulated radiotherapy [IMRT] or volumetric-modulated arc therapy [VMAT] vs non-IMRT or non-VMAT), disease stage at screening and planned total radiotherapy dose. The primary endpoints were PFS per RECIST v1.1, assessed by the investigator or histopathologic confirmation and OS. Key secondary endpoints included 24-month PFS, ORR, PROs and safety.
The study met its primary endpoint.24 At a median follow-up of 17.9 months, pembrolizumab plus CRT showed a statistically significant improvement in PFS versus placebo plus CRT (Figure 3). Furthermore, the 24-month PFS rates were 67.8% with pembrolizumab plus CRT versus 57.3% with placebo plus CRT, with a median PFS not reached in either group (HR: 0.70 [95% CI: 0.55–0.89]; p=0.002). Consistent PFS benefits were observed across all prespecified patient subgroups, including age, race, ECOG PS score, type of external beam radiotherapy, disease stage and total radiotherapy dose.
OS data were not mature at the data cut-off, representing only 42.9% of the deaths expected in the final analysis.24 A trend towards increased OS was observed in the pembrolizumab arm versus the placebo arm. At 24 months, the OS rates were 87.2% versus 80.8% with pembrolizumab versus placebo (HR: 0.73 [95% CI: 0.49–1.07]); however, these data did not cross the boundary of statistical significance. A numerically better ORR was observed in the pembrolizumab arm versus the placebo arm (79.3% vs 75.9%). The DoR, which was an exploratory endpoint of the study, also favored pembrolizumab, with 81.4% of patients versus 77.3% with placebo still being in response at 12 months.
With respect to safety, 96% of patients in both groups experienced TRAEs.24 The rates of grade ≥3 and serious TRAEs were 67.0% versus 60.6% and 17.2% versus 12.3% with pembrolizumab versus placebo, respectively. Only one all-cause AE not related to treatment and leading to treatment discontinuation was reported with pembrolizumab, and no excess deaths occurred in the experimental arm compared with placebo. The most frequently reported TRAEs were anemia, nausea and diarrhea, with similar incidence rates between the treatment arms. Immune-mediated AEs occurred more frequently in the pembrolizumab arm versus the placebo arm (any grade, 32.6% vs 11.7%; grade ≥3, 4.2% vs 1.1%). The most common immune-mediated AEs included hypothyroidism (19.3% vs 4.5%) and hyperthyroidism (11.4% vs 2.1%) with pembrolizumab versus placebo, respectively, with an incidence of grade ≥3 thyroid dysfunction of less than 2% in both treatment groups. No clinically meaningful differences in HQoL were observed between the groups from baseline to week 36, as assessed by EORTC QLQ-C30.
In conclusion, pembrolizumab combined with chemoradiotherapy demonstrated a statistically significant and clinically meaningful improvement in PFS and a favorable trend in OS compared with placebo plus chemoradiotherapy and had a manageable safety profile in patients with high-risk, treatment-naïve, locally advanced cervical cancer. These data suggest that pembrolizumab plus concurrent CRT can be considered as a new standard of care for locally advanced cervical cancer with PD-L1 expression. Although the final results are not yet published, the convincing data led to FDA approval of pembrolizumab in this indication.
EMPOWER-Cervical 1: Cemiplimab in advanced cervical cancer
This open-label, multicenter, phase III trial evaluated the anti-PD-1 antibody cemiplimab in patients with recurrent or metastatic cervical cancer who had disease progression after first-line platinum-based chemotherapy.25 Patients were required to have measurable disease, an ECOG PS score of 0 or 1 and adequate renal, hepatic and bone marrow function. Enrollment was allowed regardless of PD-L1 expression status, and previous anti-PD-1 or anti-PD-L1 treatments were not permitted. A total of 608 women (age range, 22–87 years) underwent 1:1 randomization to receive cemiplimab or the investigator’s choice of chemotherapy selected by the investigator from protocol-specified regional options, including pemetrexed, topotecan, irinotecan, gemcitabine or vinorelbine. Participants were stratified according to histological tumor type (squamous cell carcinoma or adenocarcinoma, including adenosquamous carcinoma), geographic region and previous bevacizumab exposure. The primary endpoint was OS and the secondary endpoints were PFS and safety.
The median duration of follow-up was 18.2 months.25 In the overall population, the median OS in the cemiplimab arm was 12.0 months compared with 8.5 months in the chemotherapy arm (HR: 0.69 [95% CI: 0.56–0.84]; p<0.001). The OS benefits of cemiplimab over chemotherapy were observed in all histological subgroups. Patients treated with cemiplimab had longer PFS than those receiving chemotherapy (HR: 0.75 [95% CI: 0.63–0.89]; p<0.001). The ORR was 16.4% with cemiplimab versus 6.3% with chemotherapy in the overall population, with response rates of 18.0% and 11.0% in cemiplimab-treated patients with PD-L1 expression ≥1% and <1%, respectively. Grade ≥3 AEs were reported in 45.0% of patients in the cemiplimab group versus 53.4% in the chemotherapy group. The most common grade ≥3 AEs were anemia (12.0% with cemiplimab vs 26.9% with chemotherapy), urinary tract infection (5.0% vs 2.8%) and neutropenia (1.0% vs 9.0%).
In summary, cemiplimab significantly improved survival compared with single-agent chemotherapy, thus supporting the use of ICIs in patients with recurrent cervical cancer who progressed after platinum-based chemotherapy, regardless of PD-L1 expression status. Since the approval of pembrolizumab was based on its anti-tumor activity in PD-L1–positive tumors, cemiplimab represents a novel therapeutic option for patients with a PD-L1 CPS <1. Currently, there are no data on the potential benefits of cemiplimab in patients who have already received pembrolizumab in the first-line setting.
CALLA: Durvalumab plus chemoradiotherapy in locally advanced cervical cancer
A randomized, multicenter, double-blind, phase III CALLA trial evaluated the anti-PD-L1 antibody durvalumab in combination with standard-of-care concurrent CRT in patients with locally advanced cervical cancer.26 In total, 770 newly diagnosed, treatment-naïve patients (median age, 49 years) were randomized 1:1 to receive durvalumab or placebo in combination with and followed by chemoradiotherapy. The primary endpoint was PFS, and the key secondary endpoints were OS, safety and tolerability.
In contrast to KEYNOTE-A1824 which studied pembrolizumab with CRT in the same disease setting, CALLA did not achieve statistical significance for its primary endpoint of prolonging PFS at a median follow-up of 18.5 months (HR: 0.84 [95% CI: 0.65–1.08]; p=0.174).26 Grade 3−4 AEs occurred in 51.7% and 51.0% of patients in the durvalumab plus CRT and placebo plus CRT arms, respectively. The negative results of the trial compared to KEYNOTE-A18 could be explained by the inclusion of high-risk patients (mostly stage IIB or IIIB with nodal involvement) in the KEYNOTE-A18 population and may be caused by a difference in the mechanism of action between PD-1-targeting pembrolizumab and PD-L1-targeting durvalumab.
Antibody-drug conjugates
ADCs represent a novel class of targeted therapeutics designed to harness the specificity of mAbs that precisely bind to tumor-associated antigens while simultaneously delivering potent cytotoxic drugs directly to cancer cells. ADCs have emerged as a promising avenue for the treatment of different types of malignancies, including gynecological cancers, demonstrating the potential to enhance therapeutic efficacy while minimizing systemic toxicity.27–29 Recently, two ADCs have been investigated in cervical cancer. Tisotumab vedotin (TV) is a first-in-class ADC targeting tissue factor, a membrane protein playing the central role in the initiation of blood coagulation which is frequently overexpressed in many solid tumors.30 The TV molecule is comprised of a humanized anti-tissue factor mAb conjugated to cytotoxic monomethyl auristatin E (MMAE) payload via a protease-cleavable valine-citrulline peptide linker.31
Trastuzumab deruxtecan (T-DXd)32 is an ADC composed of a cytotoxic payload deruxtecan, a derivative of a topoisomerase I inhibitor DX-8951, and a humanized mAb trastuzumab targeting human epidermal growth factor receptor 2 (HER2) which plays a key role in the pathogenesis of several cancer types and is frequently expressed in cervical cancer.33
innovaTV 204: Tisotumab vedotin in recurrent or metastatic cervical cancer
The pivotal, multicenter, open-label, single-arm, phase II innovaTV 204 trial assessed TV in patients with previously treated recurrent or metastatic cervical cancer.34 The enrollment criteria included disease progression on or after doublet chemotherapy with bevacizumab, ≤2 previous systemic therapies for recurrent or metastatic disease and measurable disease per RECIST v1.1 and an ECOG PS score of 0 or 1. Patients (n=102, age ≥18 years) received TV until disease progression or unacceptable toxicity. The primary endpoint was confirmed ORR.
At a median follow-up of 10.0 months, TV demonstrated a confirmed ORR of 24%, including 7% CRs.34 The most frequent TRAEs included alopecia (38%), epistaxis (30%), nausea (27%), conjunctivitis (26%), fatigue (26%) and dry eye (23%). Grade ≥3 TRAEs were observed in 28% of the patients, including neutropenia, fatigue, ulcerative keratitis and peripheral neuropathies. One death due to septic shock was considered to be related to therapy. Based on clinically meaningful and durable antitumor activity, the FDA granted accelerated approval to TV for the treatment of recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.35
innovaTV 301: Tisotumab vedotin in recurrent or metastatic cervical cancer
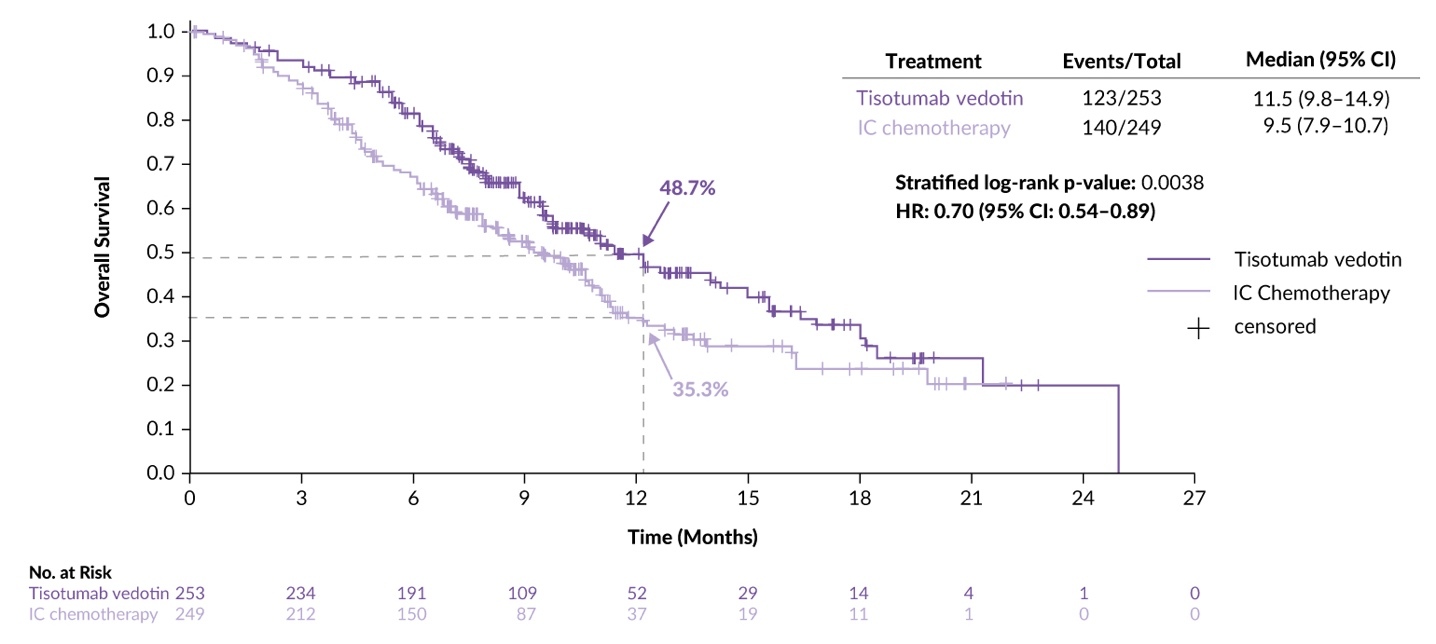
A recent presentation at the ESMO 2023 Congress reported a planned interim analysis of innovaTV 301, a randomized, open-label, phase III trial that investigated the efficacy and safety of TV versus the investigator’s choice of chemotherapy in patients with recurrent or metastatic cervical cancer.36 The study enrolled patients (median age, 50 years) with disease progression on or after a standard-of-care doublet chemotherapy regimen with or without bevacizumab and anti-PD-(L)1 therapy, after ≤2 prior therapy lines, measurable disease per RECIST v1.1 and an ECOG PS score of 0−1. A total of 502 patients were randomized 1:1 to receive TV monotherapy (n=253) or chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan or pemetrexed). The primary endpoint was OS and the key secondary endpoints included PFS, confirmed ORR and safety. In the TV and chemotherapy arms, 64.8% and 63.1% of patients received prior bevacizumab therapy and 28.1% and 26.9% received prior anti-PD-(L)1 therapy, respectively. The median follow-up was 10.8 months.
The efficacy analysis demonstrated that patients in the TV arm had a 30% reduction in the risk of death compared with those in the chemotherapy arm (HR: 0.70 [95% CI: 0.54–0.89]; p=0.0038), with a median OS of 11.5 months versus 9.5 months, respectively (Figure 4). Furthermore, PFS was superior with TV compared to chemotherapy (HR: 0.67 [95% CI: 0.54–0.82]; p<0.0001), with a median PFS of 4.2 months versus 2.9 months. OS and PFS benefits were generally consistent across key subgroups, including prior bevacizumab and anti-PD-(L)1 therapy, as well as the number of prior treatment lines. The confirmed ORR was 17.8% and 5.2% in the TV and chemotherapy arms, respectively (odds ratio: 4.0 [95% CI: 2.1–7.6]; p<0.0001).
Most patients in the study experienced TRAEs (TV, 87.6% [grade ≥3, 29.2%]; chemotherapy, 85.4% [grade ≥3, 45.2%]).36 Grade 5 TRAEs occurred in 2 (0.8%) and 1 (0.4%) patients in the TV and chemotherapy arms, respectively. The safety profile for TV was consistent with previous reports, including AESIs, such as ocular events, peripheral sensory neuropathy and bleeding. Dose discontinuations due to ocular and peripheral neuropathy events were reported in 5.6% of patients for each condition.
In conclusion, TV showed a statistically significant and clinically meaningful improvement in OS, PFS and ORR versus chemotherapy, with a tolerable safety profile in patients with previously treated recurrent or metastatic cervical cancer, suggesting that TV should be considered as a potential new standard of care for patients who have progressed after one line of systemic therapy. The study population, which comprised patients who had progressed on pembrolizumab and bevacizumab, was particularly noteworthy in achieving a positive outcome, as it fulfilled the unmet need for effective treatments in the current treatment situation.
DESTINY-PanTumor: Trastuzumab deruxtecan in cervical cancer
The open-label, phase II DESTINY-PanTumor basket study investigated T-DXd in several types of HER2-expressing solid tumors.37 The study population included 267 patients (median age, 62 years), of which 40 had cervical cancer. The primary endpoint was confirmed ORR and secondary endpoints included DoR, disease control rate (DCR), PFS, OS and safety.
The efficacy analysis demonstrated high clinical activity of T-DXd, with the best responses achieved in endometrial and cervical tumors.37 At a median follow-up of 7.2 months in the cervical cancer cohort, ORR per investigator was 50% (including 5% of CRs), with an ORR of 75% and 40% in the subgroups with HER2 expression status of IHC 3+ and IHC 2+, respectively. DCR was 67.5% at 12 weeks, and the median DoR was 9.8 months. The toxicity profile of T-DXd was manageable, with 38.6% of patients in the overall population experiencing grade ≥3 TRAEs, including neutropenia (19.1%) and anemia (8.6%). AESIs for T-DXd included interstitial lung disease/pneumonitis and left ventricular dysfunction (grade ≥3, 0.4% in each case). Although the study population was relatively small, these data may be practice-changing for gynecological cancers, including cervical cancer.
Induction chemotherapy before chemoradiation
CRT has been the standard of care in locally advanced cervical cancer for more than two decades. Despite significant progress in radiotherapy techniques and refinements in radiation dose and treatment duration, a considerable number of patients continue to experience disease relapses, resulting in a poor prognosis.1,6 Neoadjuvant chemotherapy administered before CRT may reduce the tumor size and prevent the development of micrometastases. However, clinical trials have yielded conflicting results showing either high response rates and significant survival advantages of induction chemotherapy or its detrimental effects on survival,38,39 with data heterogeneity being likely due at least partly to differences in trial design and treatment regimens.
INTERLACE: Induction chemotherapy followed by chemoradiation in locally advanced cervical cancer
The multicenter, phase III INTERLACE study performed by the Gynecologic Cancer Intergroup (GCIG) aimed to investigate whether short-course induction chemotherapy delivered before standard CRT improves PFS and OS in patients with newly diagnosed squamous, adeno- or adenosquamous carcinoma (FIGO stage IB1 node-positive, IB2, II, IIIB and IVA).40 In total, 500 women (median age, 46 years) were randomized 1:1 to receive either standard CRT with cisplatin alone or induction chemotherapy (6 weeks of carboplatin plus paclitaxel) followed by standard CRT. Patients were stratified according to disease stage, site, tumor size and histology, nodal status and type of radiotherapy and brachytherapy. The primary endpoints were PFS (target HR: 0.65) and OS (target HR: 0.65−0.70).
The study met its primary endpoint.40 At a median follow-up of 64 months, 5-year PFS rates were 73% in the induction chemotherapy plus CRT arm compared with 64% in the CRT arm (HR: 0.65 [95% CI: 0.46–0.91]; p=0.013) corresponding to a 9% difference in PFS in favor of induction chemotherapy (Figure 5). Furthermore, an 8% improvement in OS was observed in the induction chemotherapy plus CRT arm, with 5-year OS rates of 80% versus 72% with induction chemotherapy plus CRT versus CRT alone (HR: 0.61 [95% CI: 0.40–0.91]; p=0.04). Notably, 70% of the patients had stadium FIGO IIB. Grade ≥3 AEs were reported in 59% and 48% of the patients who received induction chemotherapy plus CRT and CRT alone, respectively. Any-grade hematologic AE rates were higher in the induction chemotherapy plus CRT arm (30% vs 13% with CRT alone), but this did not compromise radiotherapy delivery.
In summary, induction chemotherapy with carboplatin plus paclitaxel, followed by CRT with cisplatin, significantly improved survival and may be considered a new standard of care for locally advanced cervical cancer.
Conclusions
Novel therapeutic approaches are urgently needed for patients with cervical cancer who experience disease progression on standard-of-care treatments. The results of recent clinical trials demonstrate that targeted therapies, including ADCs and ICIs, will play a critical role in the treatment of locally advanced, as well as metastatic, recurrent or persistent cervical cancer. They are effective as second-line and first-line treatments and can be used either as monotherapy or in combination with chemotherapy and radiotherapy. Together with continuous efforts aimed at optimizing chemotherapy and radiotherapy regimens, these promising strategies offer new hope to patients with cervical cancer.
Conflict of interest
The authors have declared that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
The authors have created and approved the final manuscript.
_with_or_withou.jpeg)
_of_patients_treated_with_atezolizumab_(atezo)_plus.jpeg)
_plu.jpeg)

_of_patients_treated_with_induction_chemotherapy.jpeg)
_with_or_withou.jpeg)
_of_patients_treated_with_atezolizumab_(atezo)_plus.jpeg)
_plu.jpeg)
_of_patients_treated_with_induction_chemotherapy.jpeg)